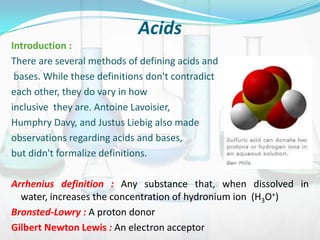
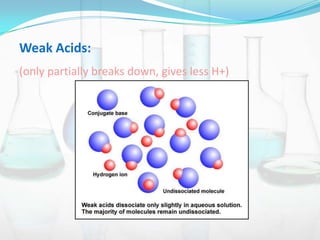
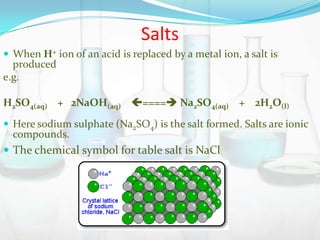
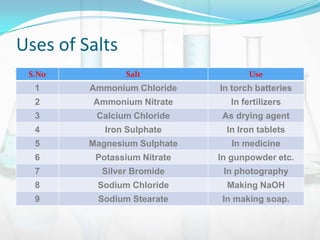
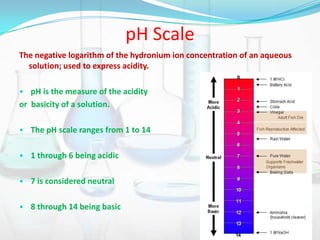
This document provides information about acids, bases, salts, and pH scale. It defines acids and bases, describes their properties and classifications. It discusses the preparation and uses of acids, bases, and salts. It also explains what the pH scale measures, how it indicates acidity and basicity, and how pH values correspond to acids and bases.
















![4. Double salt
Salt that ionizes to produce three different types of ions in
solution, two of these are usually positively charged and the
other negatively charged. Examples are ammonium iron(II)
tetraoxosulphate(VI) hexahydrate, (NH4)2 Fe(SO4)2.6H2O;
potash alum or aluminium potassium tetraoxosulphate(VI)
dodecahydrate, KAl(SO4)2. 12H2O; and chrome alum or
chromium(III) potassium tetraoxosulphate(VI) dodecahydrate,
KCr(SO4)2. 12H2O.
5. Complex salt
The salt contains complex ions, i.e. ions consisting of a charged
group of atoms. Examples are sodium tetrahydroxozincate(II)
Na2Zn(OH)4(aq)2Na+(aq)+Zn(OH)2-4(aq)
potassium hexacyanoferrate(II)
K4Fe(CN)6(aq)4K+(aq)+[Fe(CN)6]4-(aq)](https://image.slidesharecdn.com/acidsbasesandsalts-131202094049-phpapp01/85/Acids-bases-and-salts-21-320.jpg)




![The pH scale ranges from 1 to 10-14 mol/L or from 1 to 14.
pH = - log [H3O+]
1 2 3 4 5 6 7 8 9 10 11 12 13 14
acid
neutral
base](https://image.slidesharecdn.com/acidsbasesandsalts-131202094049-phpapp01/85/Acids-bases-and-salts-28-320.jpg)
![Manipulating pH
Algebraic manipulation of:
pH = - log [H3O+]
allows for:
[H3O+] = 10-pH
If pH is a measure of the hydronium ion concentration
then the same equations could be used to describe the
hydroxide (base) concentration.
[OH-] = 10-pOH
pOH = - log [OH-]
thus:
pH + pOH = 14 ; the entire pH range!](https://image.slidesharecdn.com/acidsbasesandsalts-131202094049-phpapp01/85/Acids-bases-and-salts-29-320.jpg)