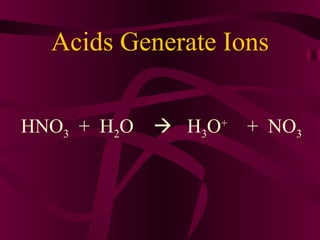
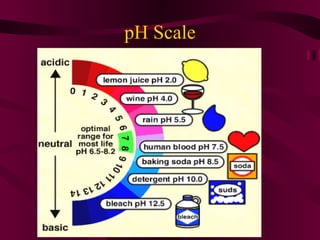
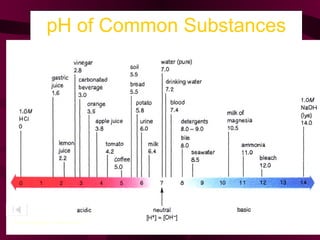
This document discusses acids, bases, and salts. It defines acids as compounds that produce hydrogen ions (H+) in water, have a pH below 7, and react with metals. Bases are defined as compounds that produce hydroxide ions (OH-) in water and have a pH above 7. Salts are neutral compounds formed by the reaction of acids and bases. Common acids and bases are listed, along with their uses. The pH scale and indicators are also explained.