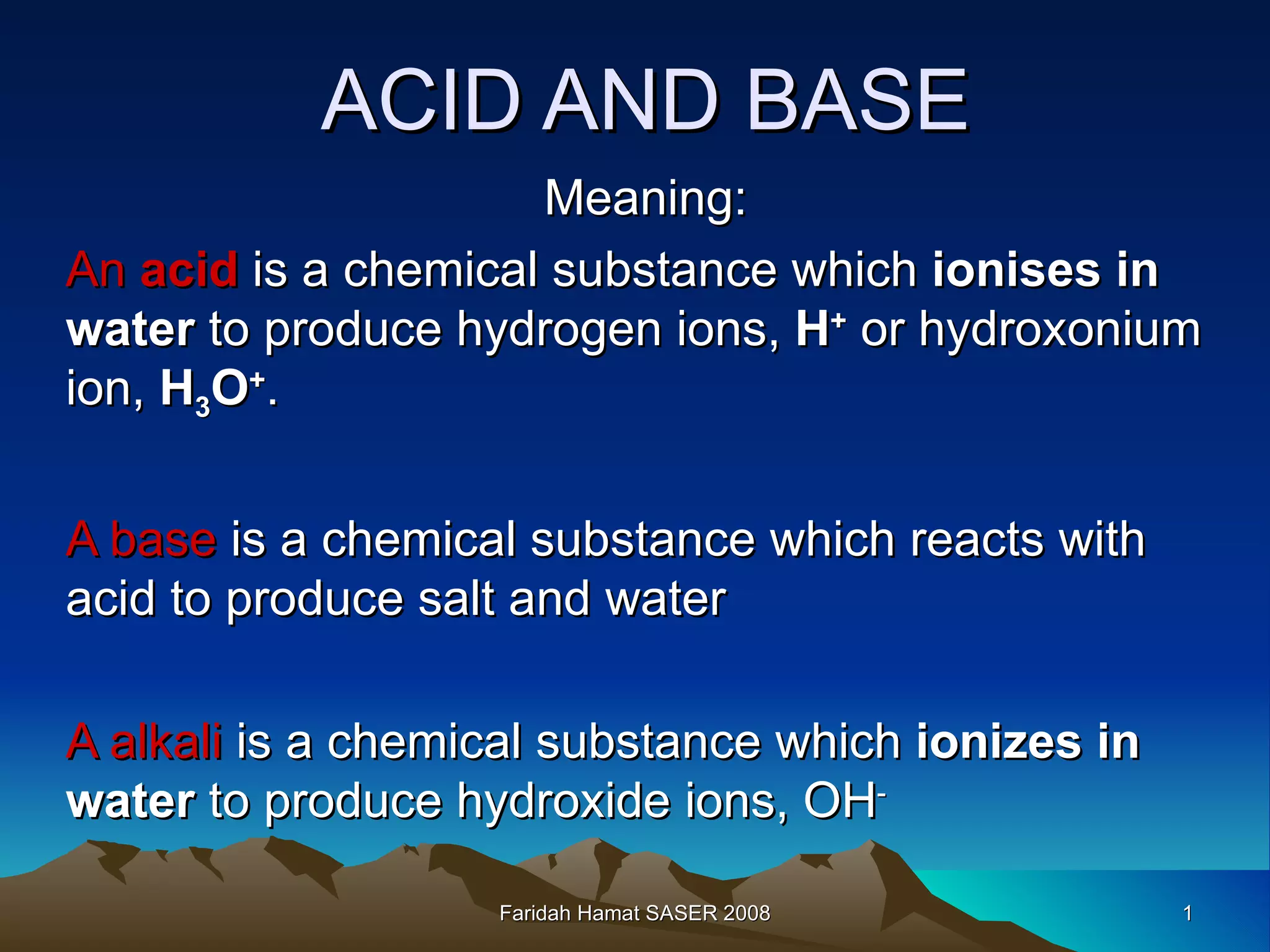
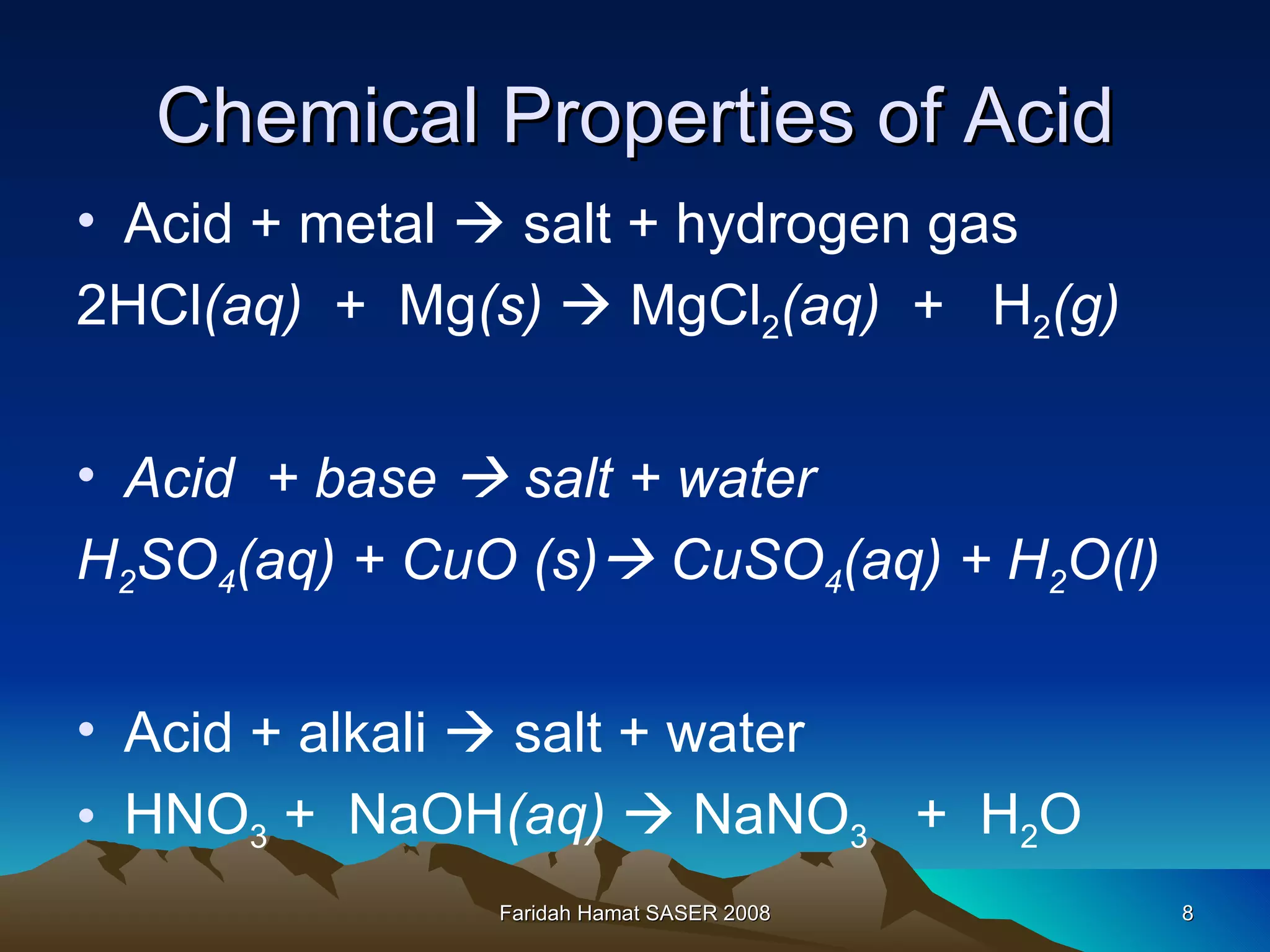
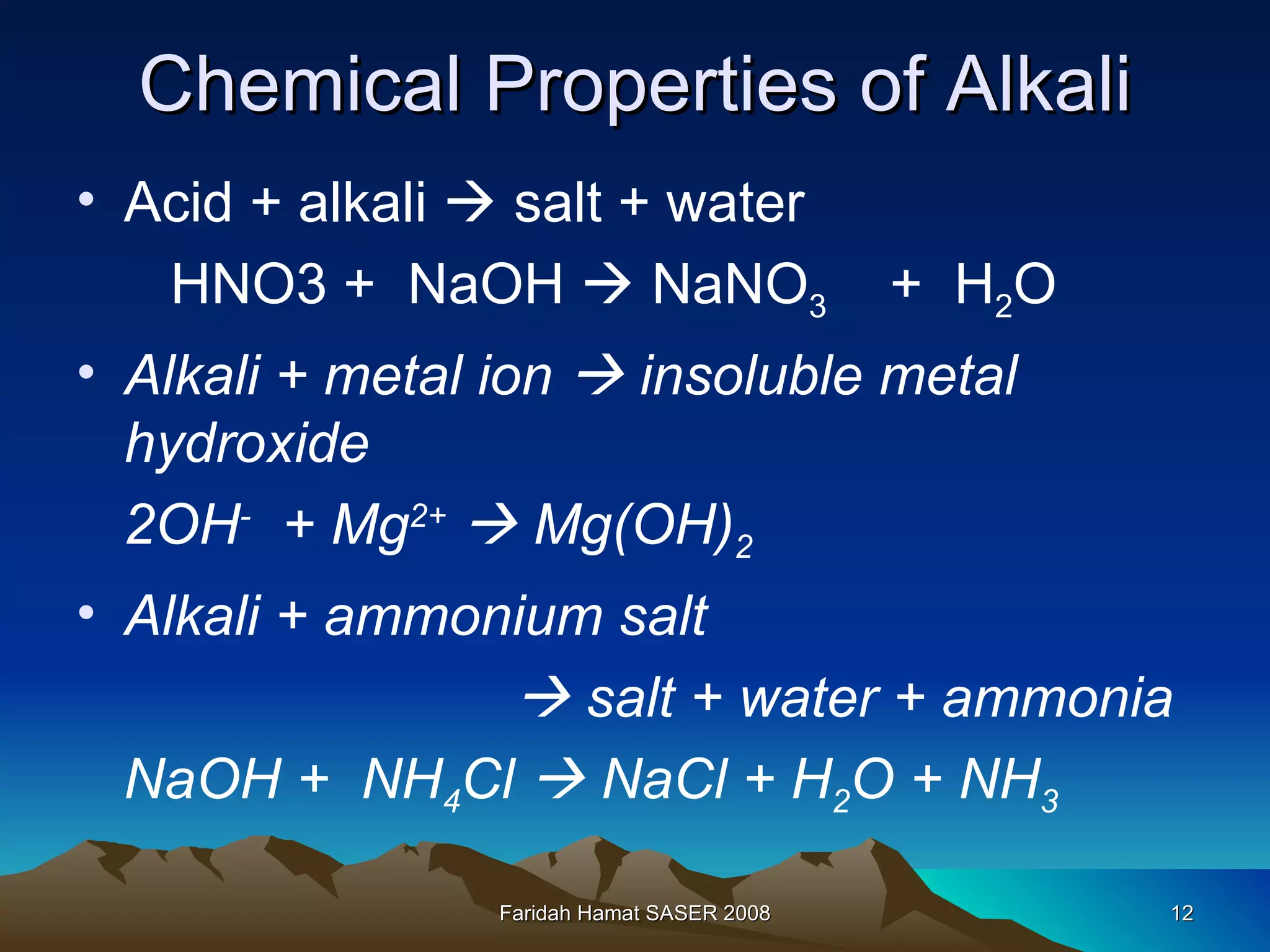
An acid is a chemical substance that produces hydrogen ions (H+) in water, while a base reacts with an acid to produce salt and water. Strong acids fully ionize in water to produce a high concentration of H+ ions, while weak acids only partially ionize, producing a low H+ concentration. Strong bases fully ionize to produce a high concentration of hydroxide (OH-) ions, unlike weak bases. Acids have sour tastes, turn litmus paper red, and are electrolytes in water. They react with metals, bases, carbonates, and alkalis to produce salts, hydrogen gas, water, and carbon dioxide. Bases have bitter tastes, turn litmus paper blue, and are electrolytes