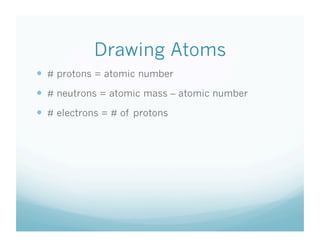
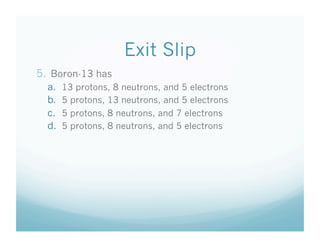
This document provides information about isotopes. It defines isotopes as atoms of the same element that have different numbers of neutrons. It discusses drawing atomic models showing protons, neutrons, and electrons. It explains that the number of protons defines the element, while the number of neutrons can vary between isotopes of the same element. The document includes sample isotope problems and exit slip questions for students to practice identifying isotopes based on atomic mass and number.