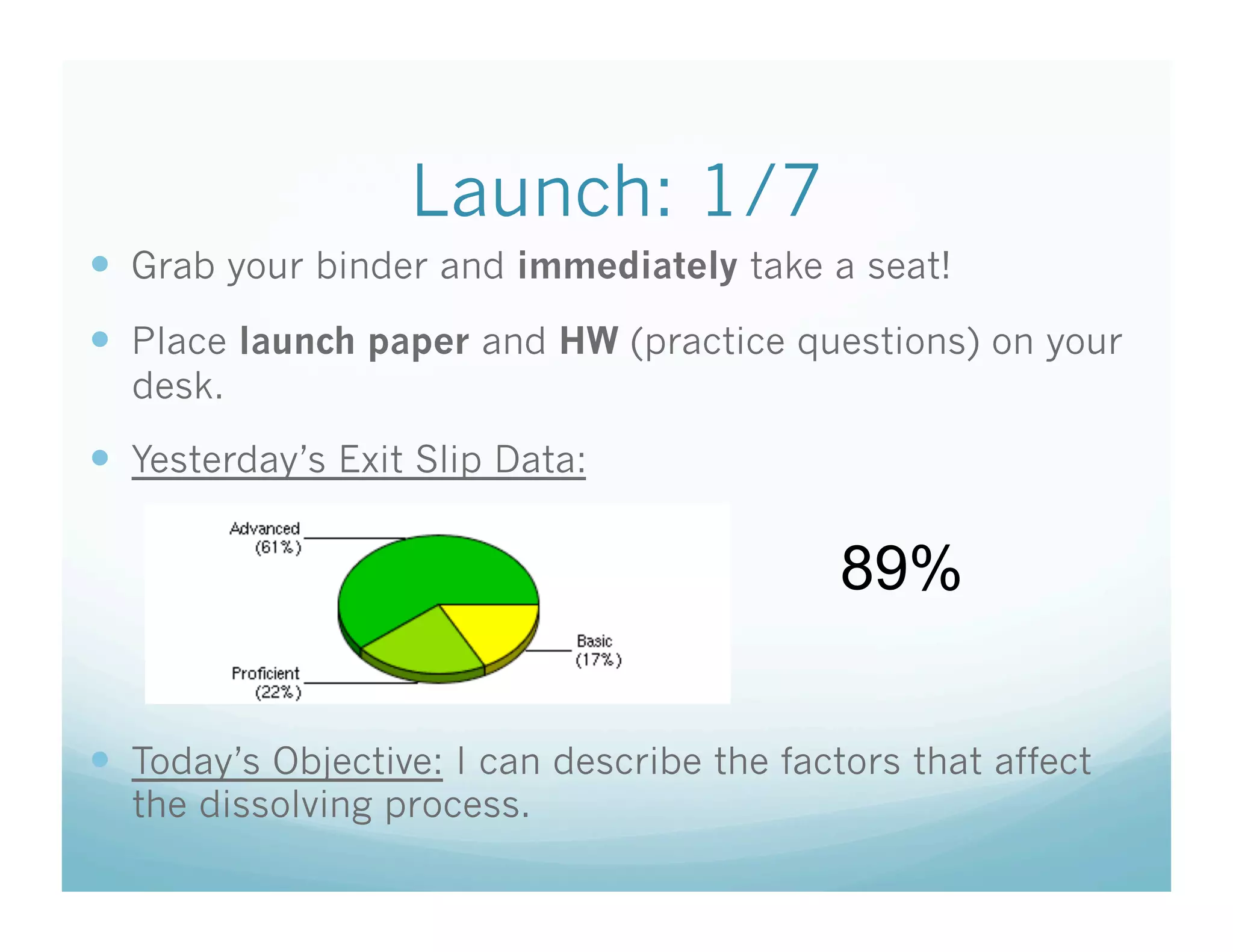
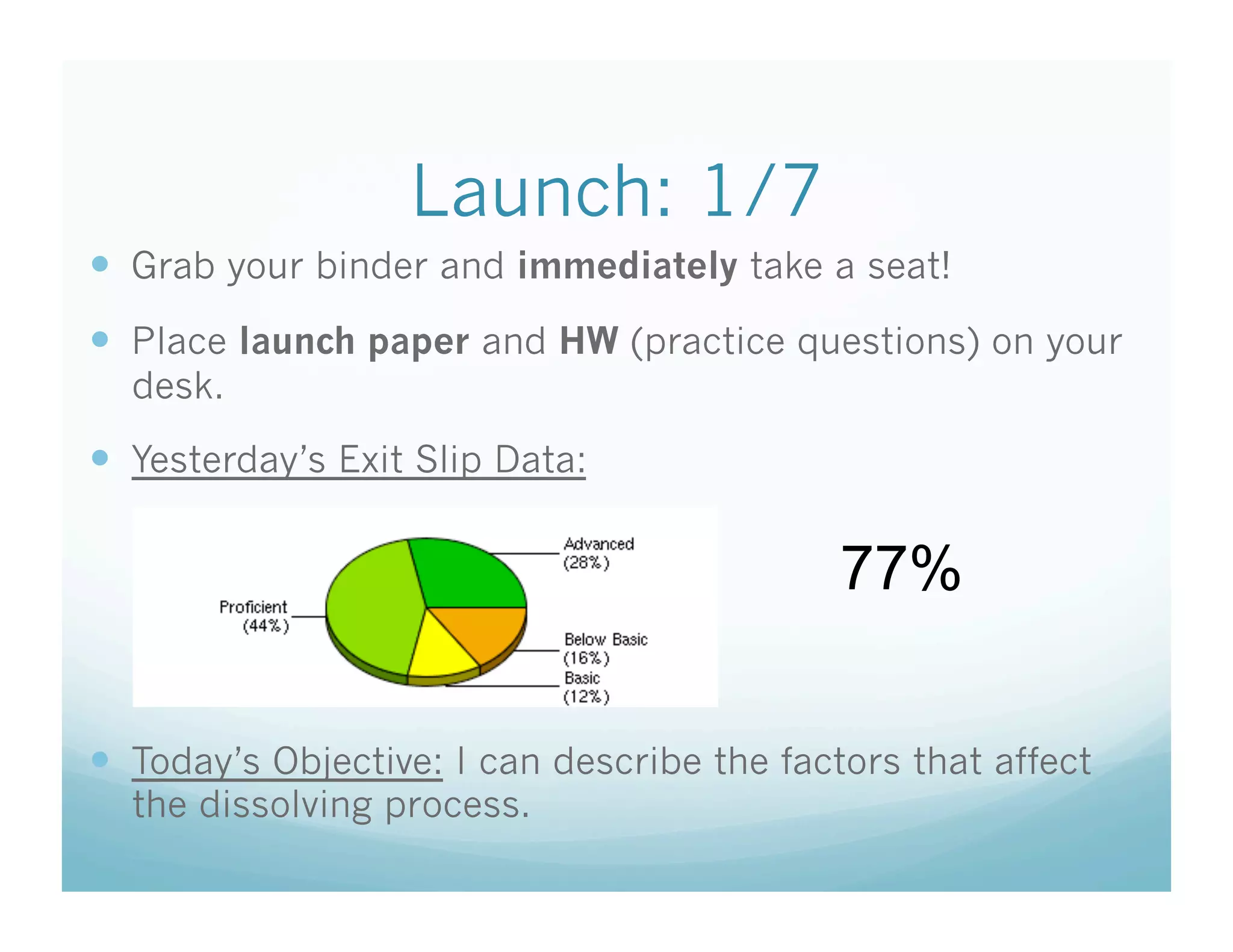
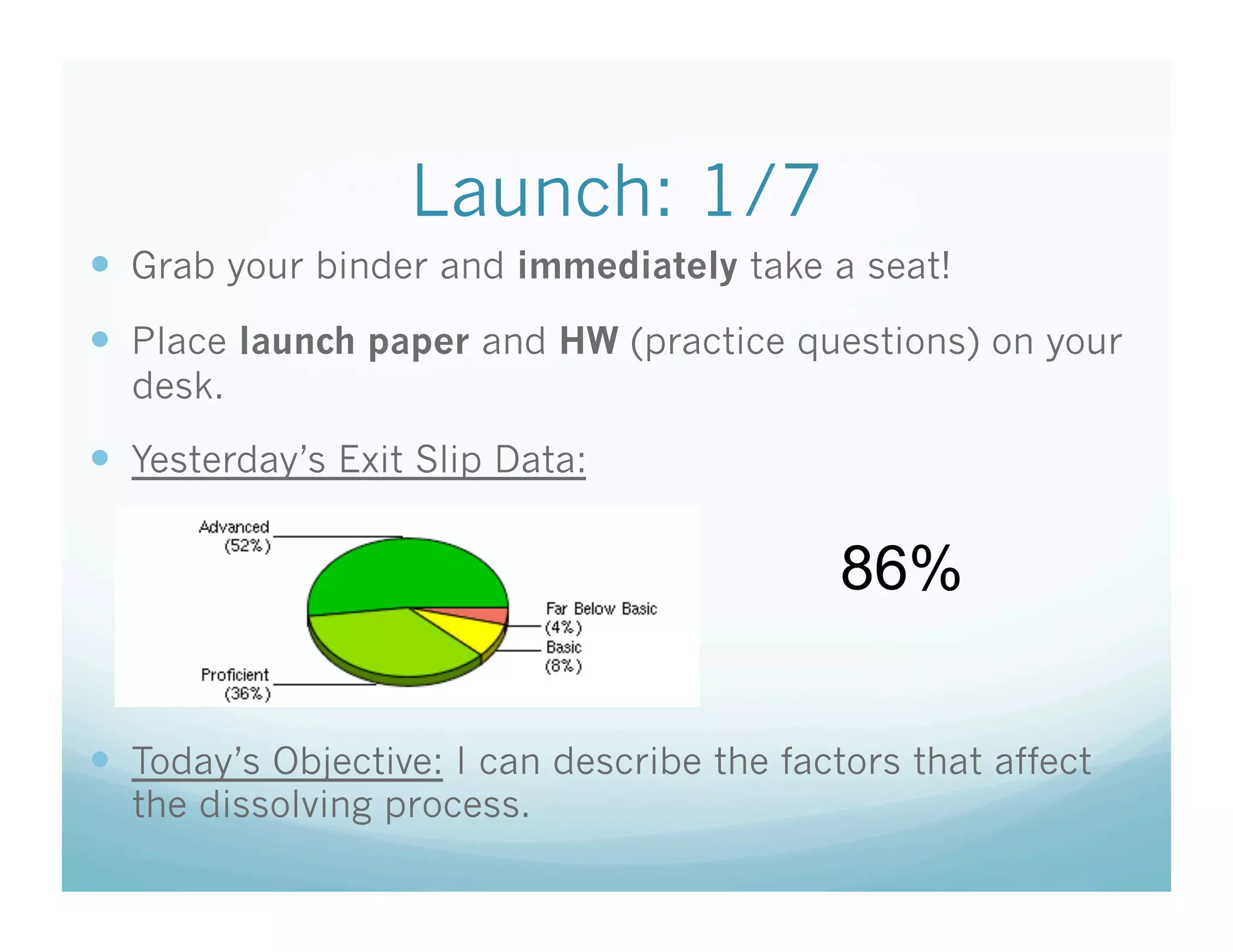
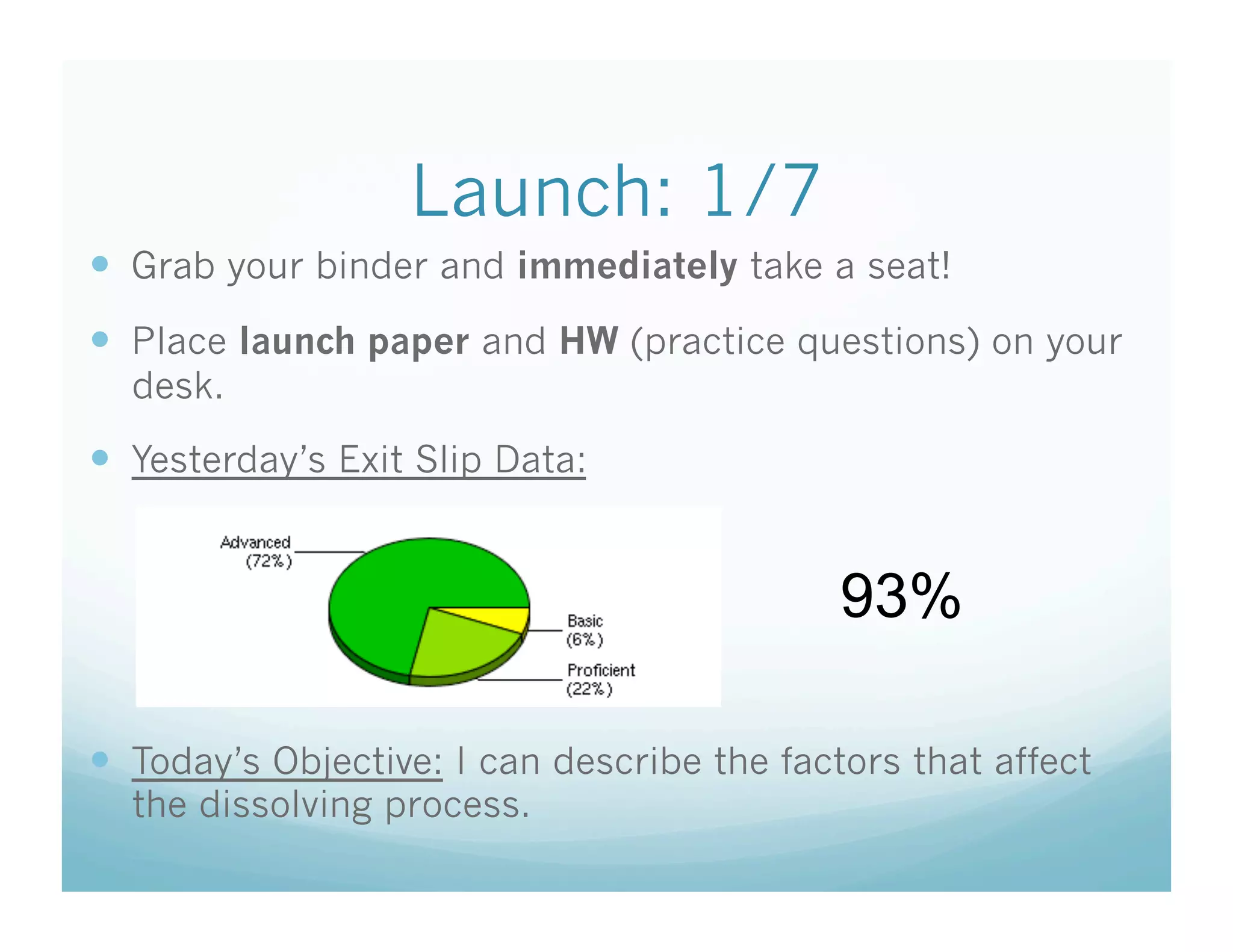
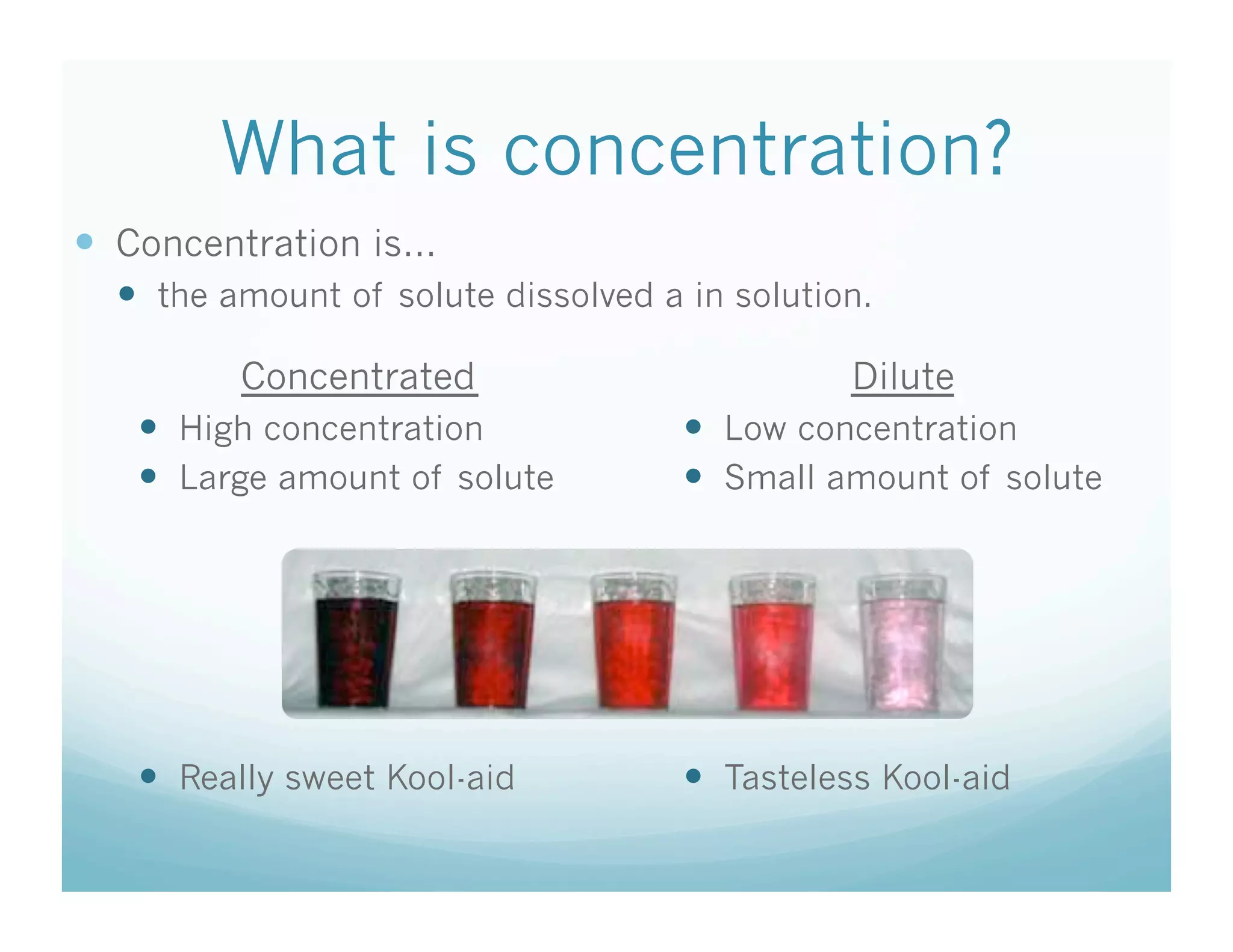
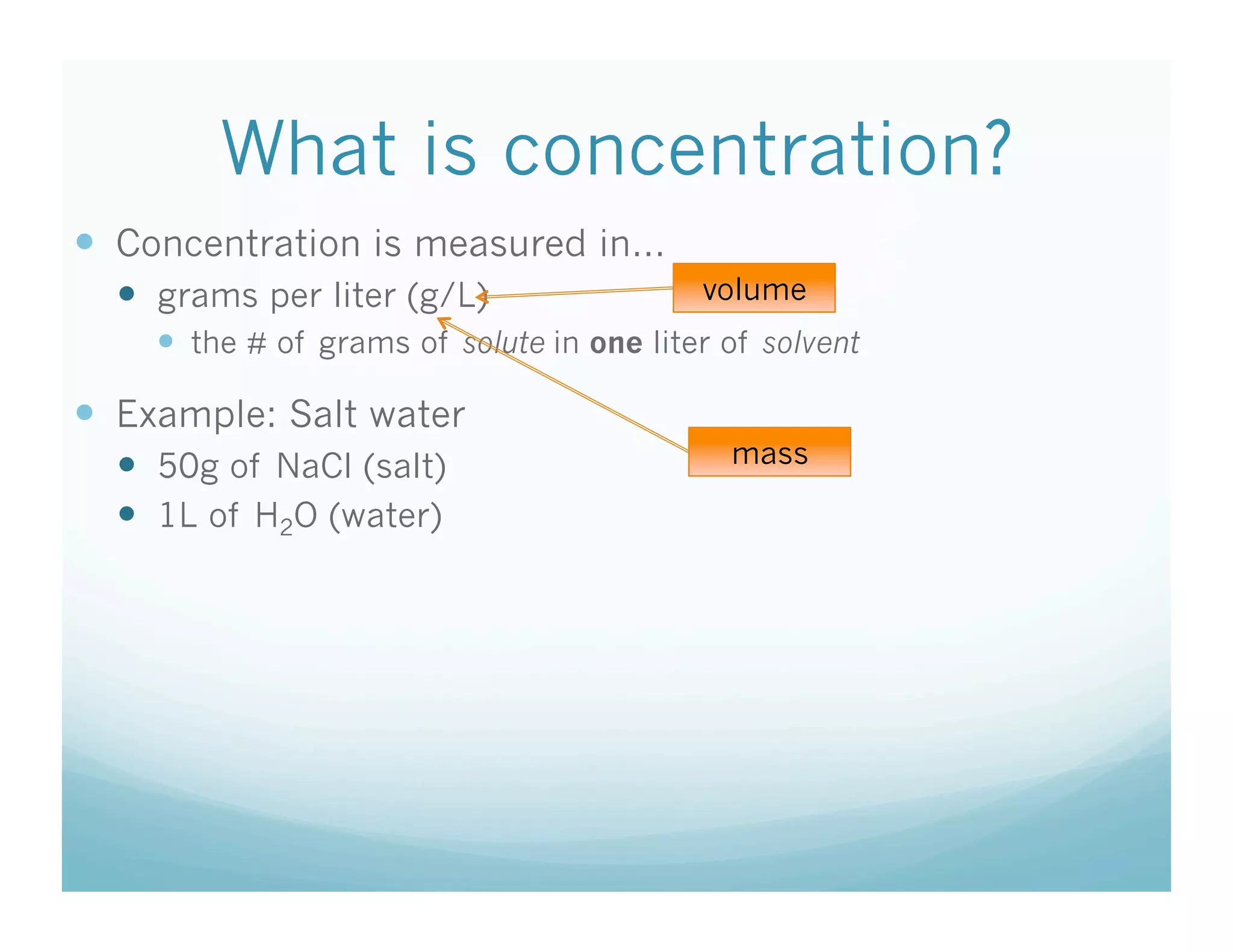
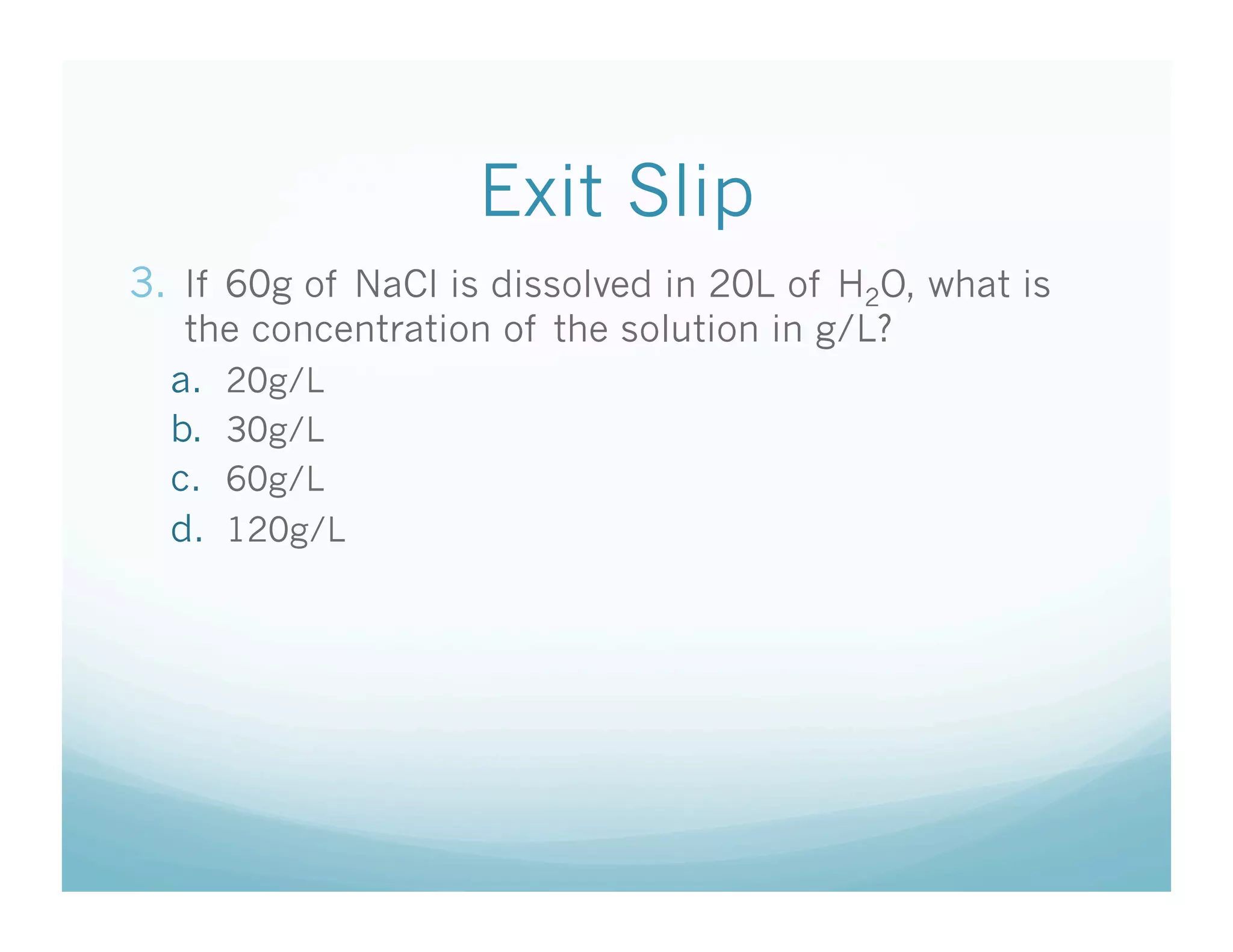
This document contains the agenda and materials for a science class lesson on concentration. The teacher provides instructions for students to take their seats and get out their homework. Exit slip data from the previous day is reported, ranging from 77-93%. The learning objective for the day is stated as being able to describe the factors that affect the dissolving process. The document then includes sample problems, examples, practice questions, and an exit slip for students to complete to assess their understanding of concentration.