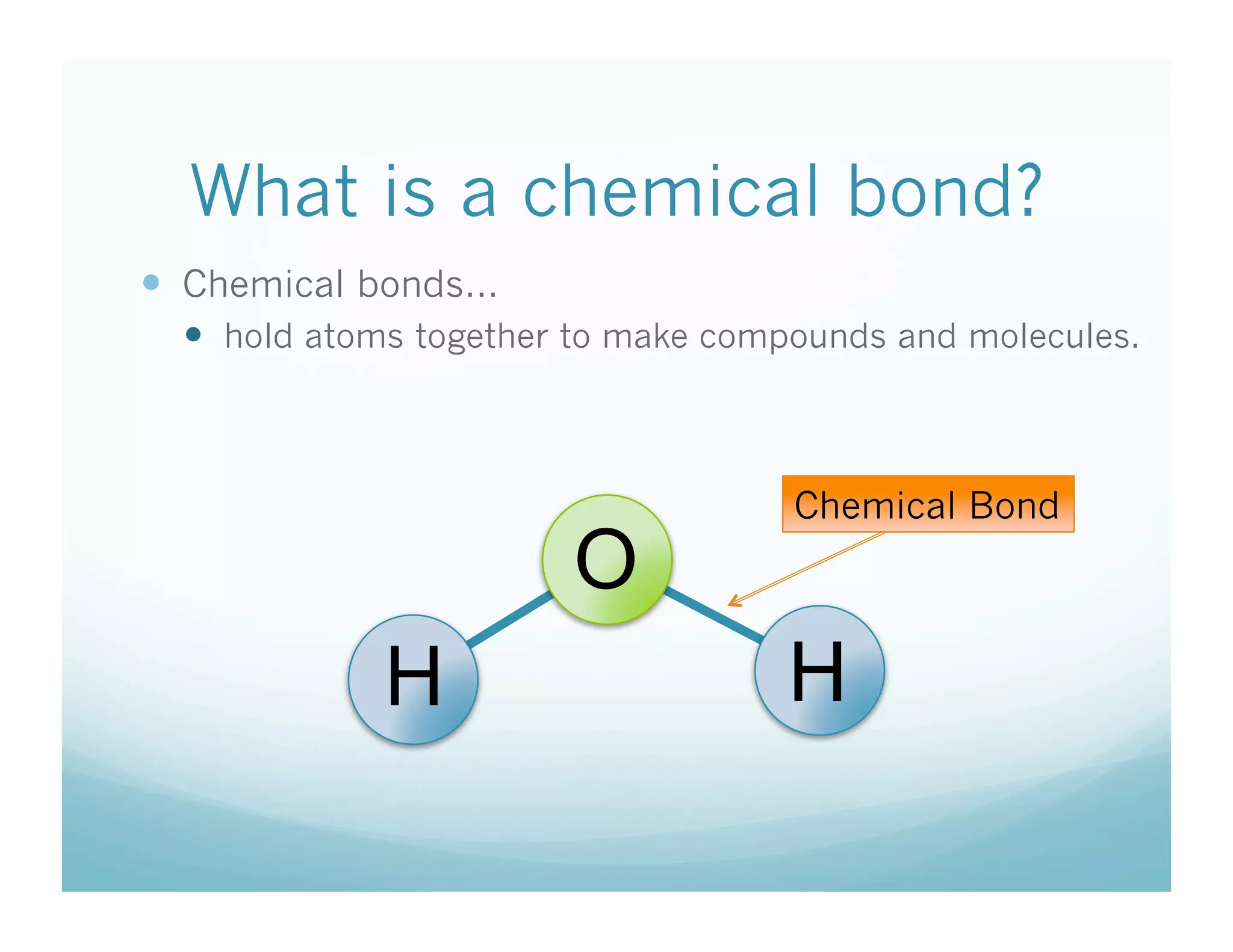
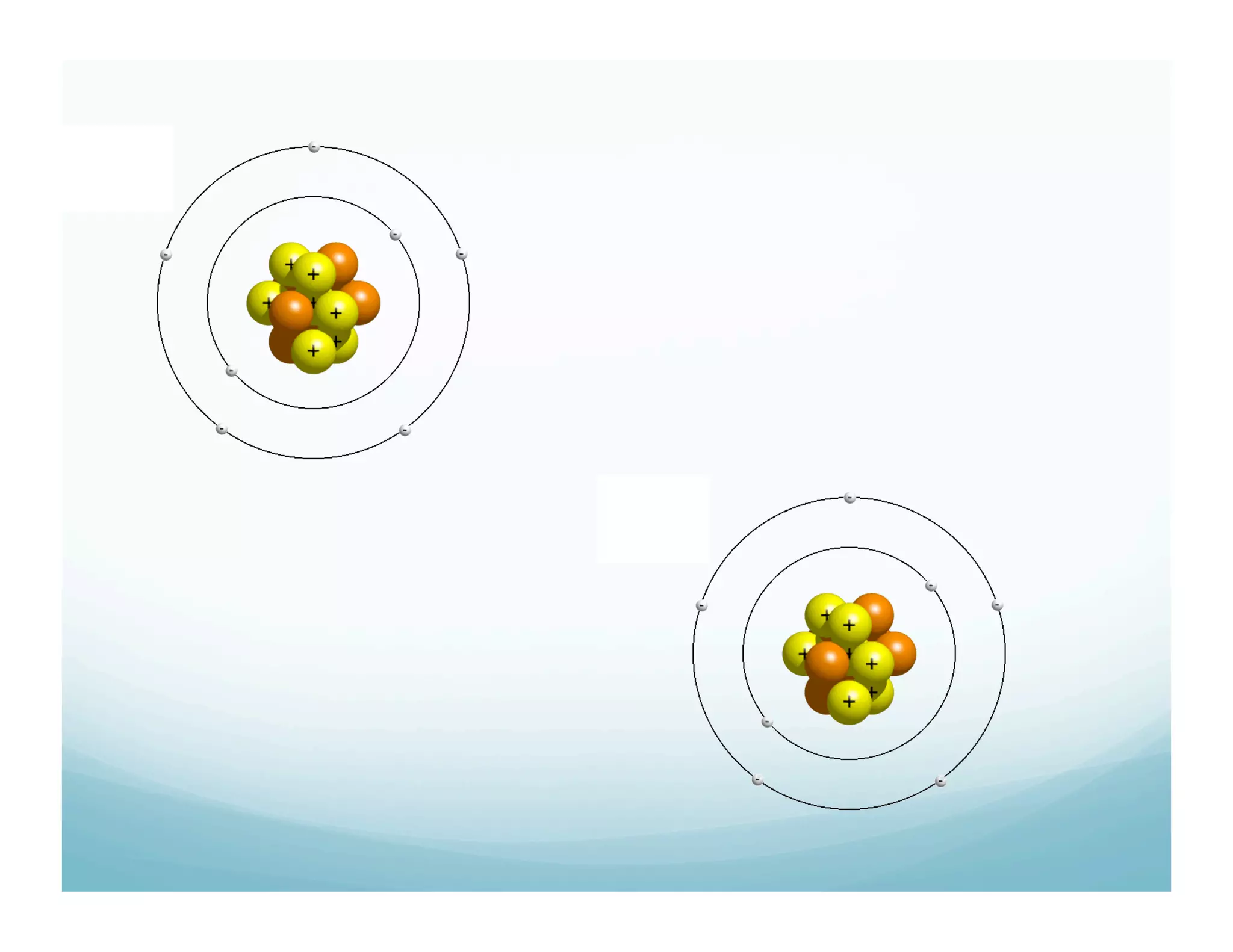
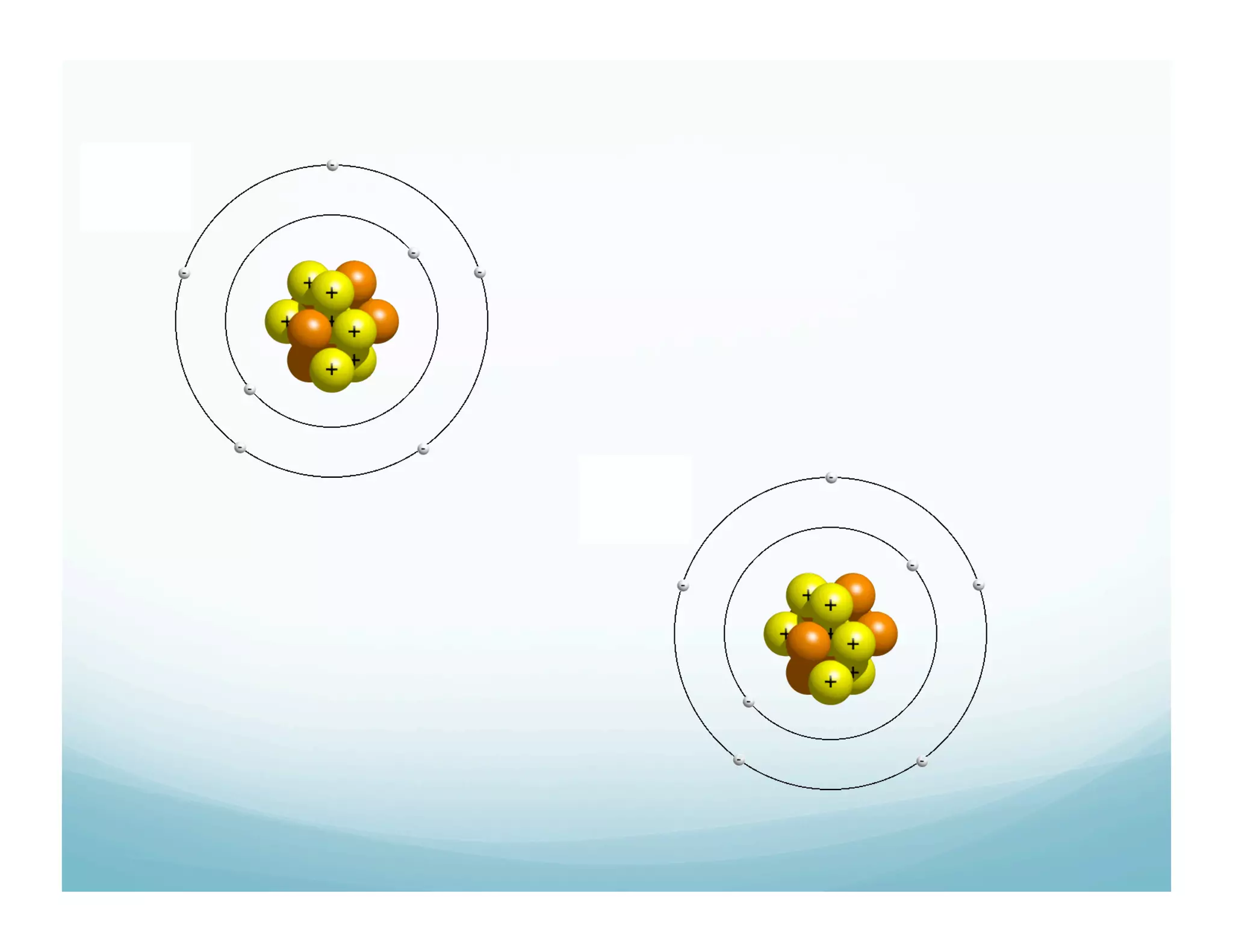
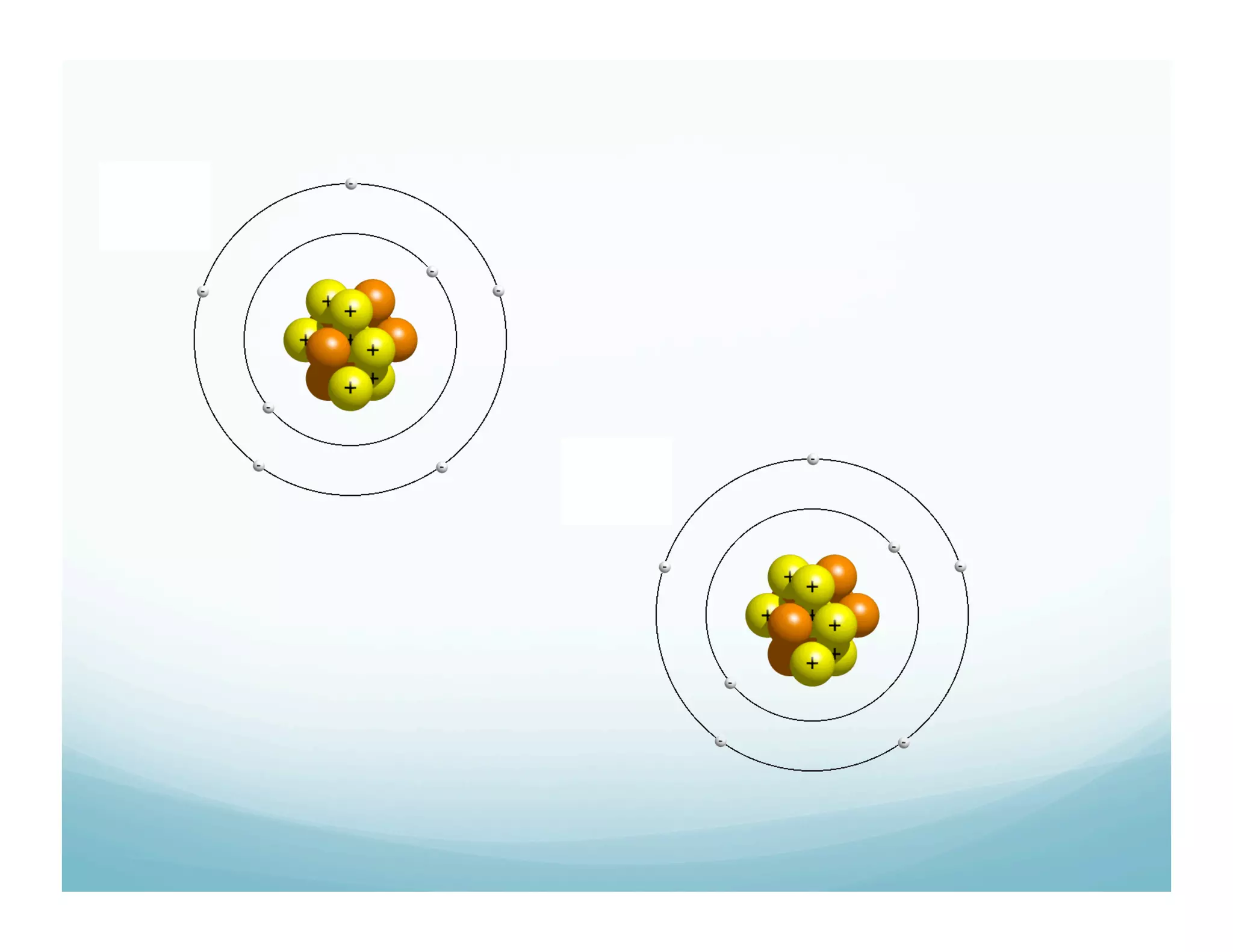
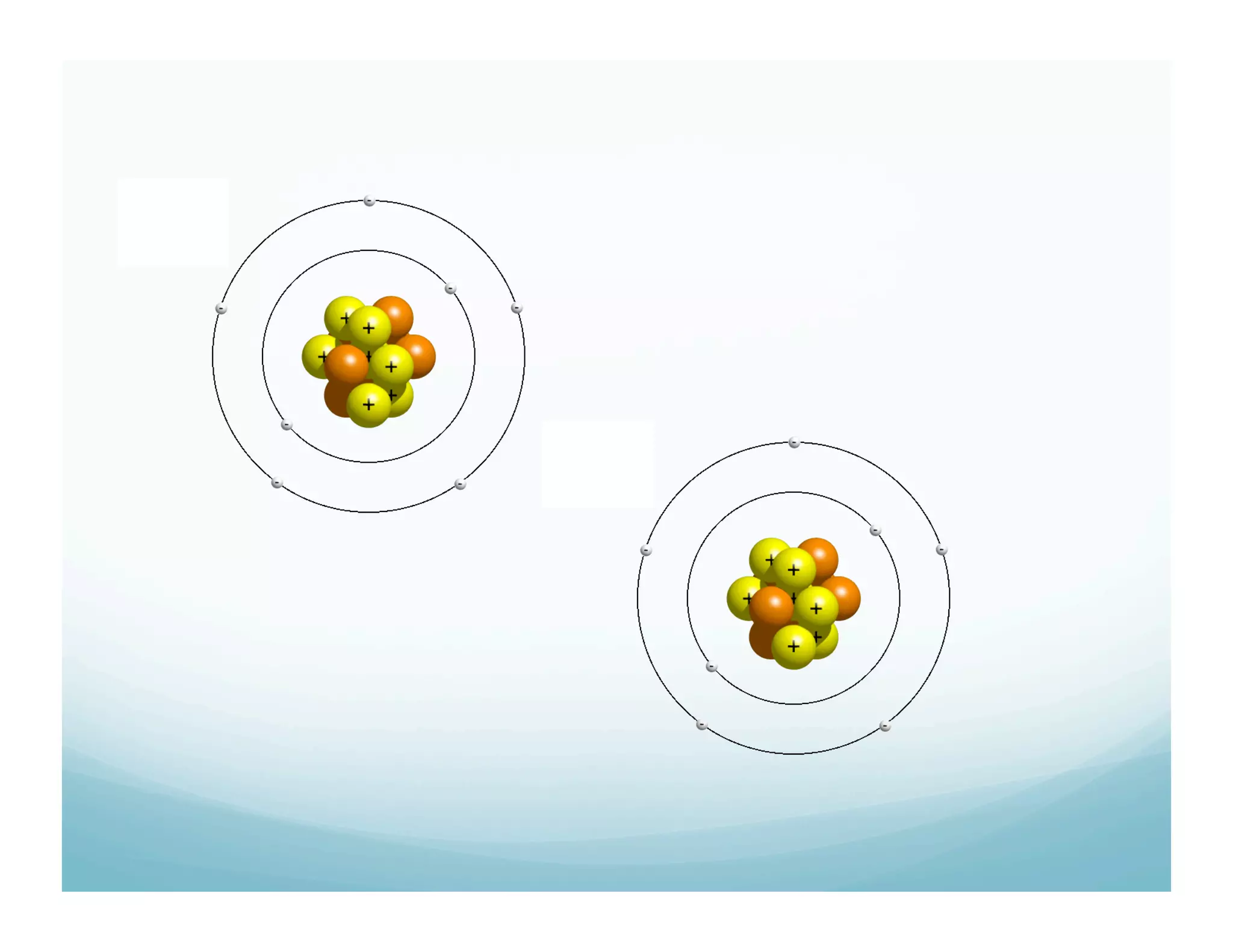
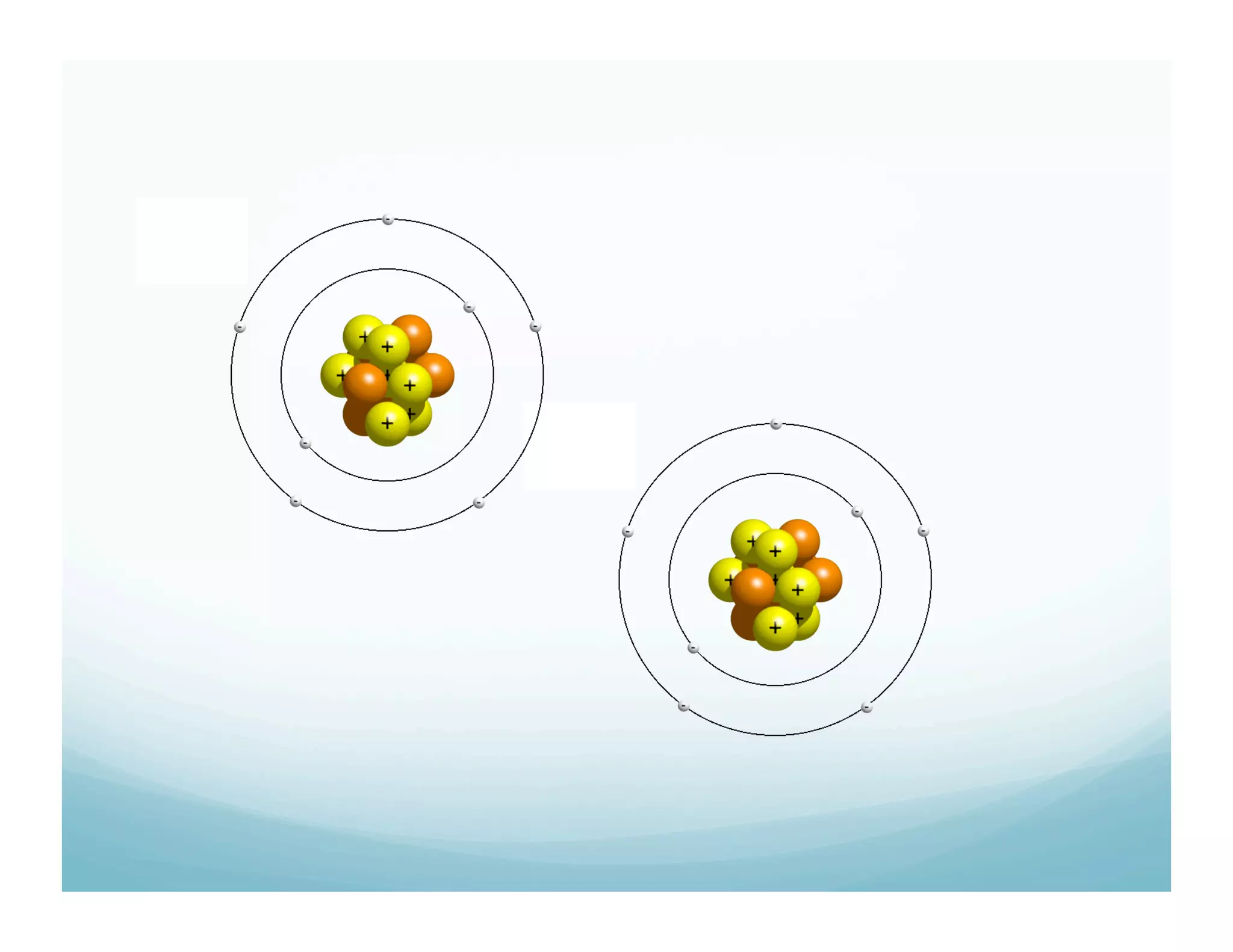
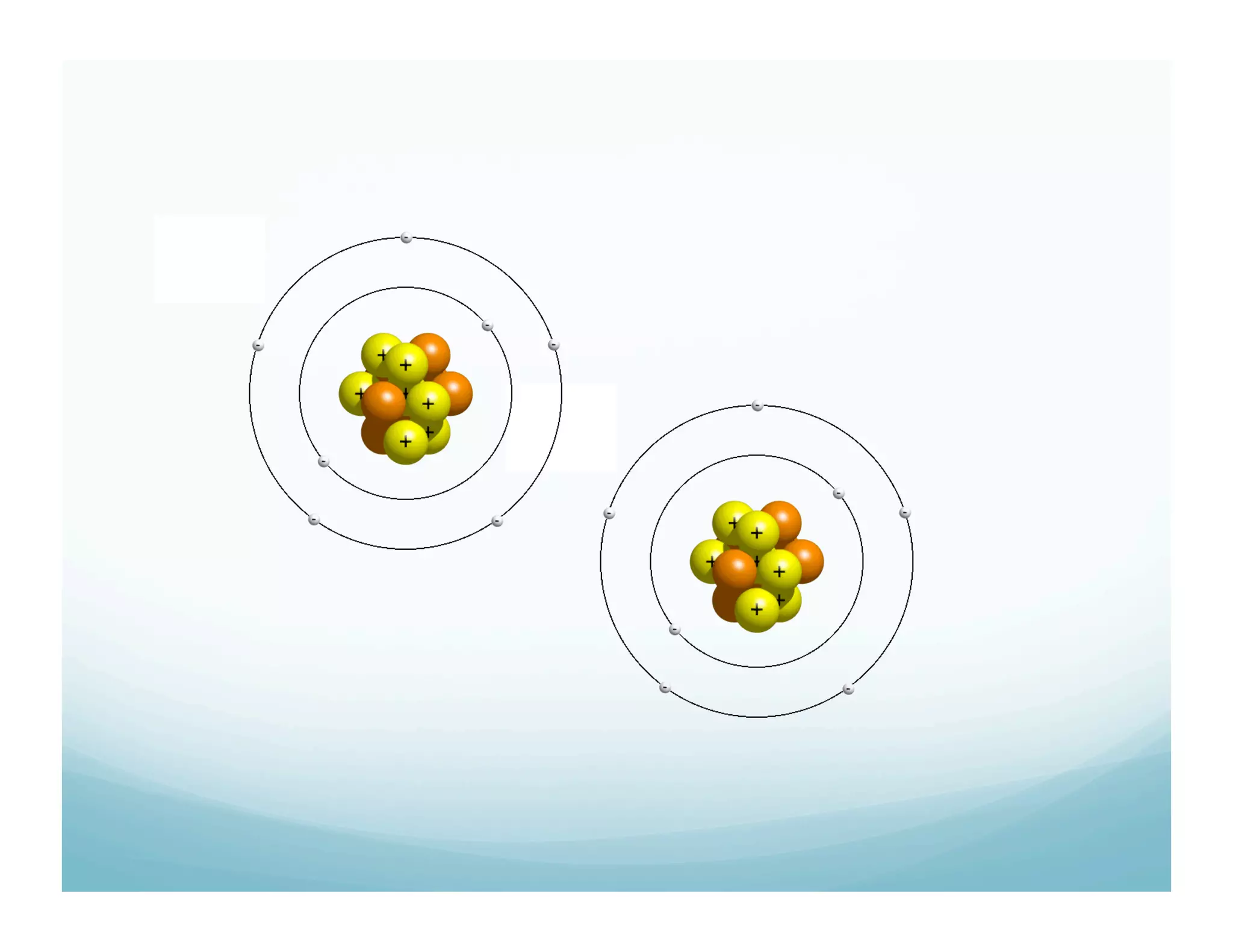
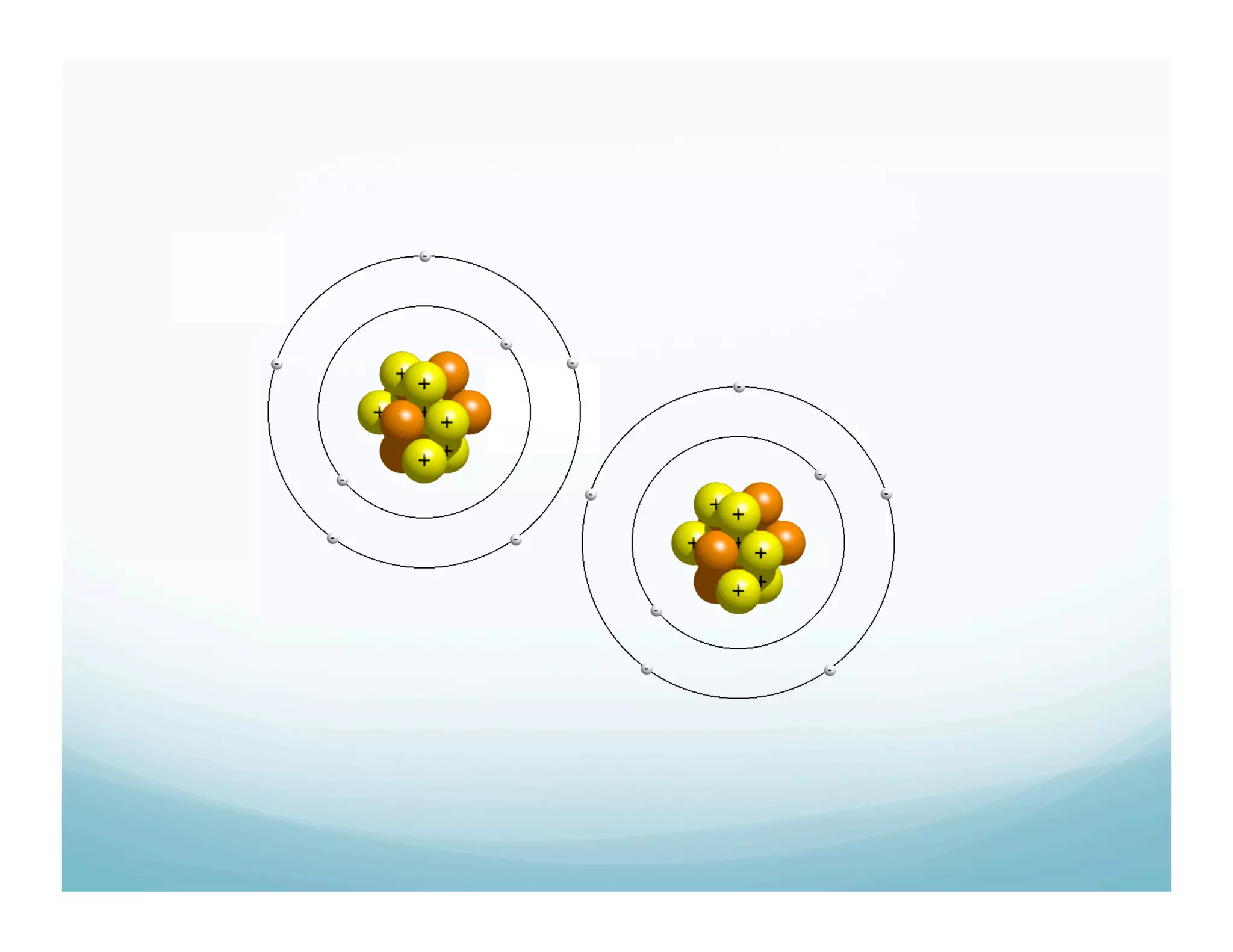
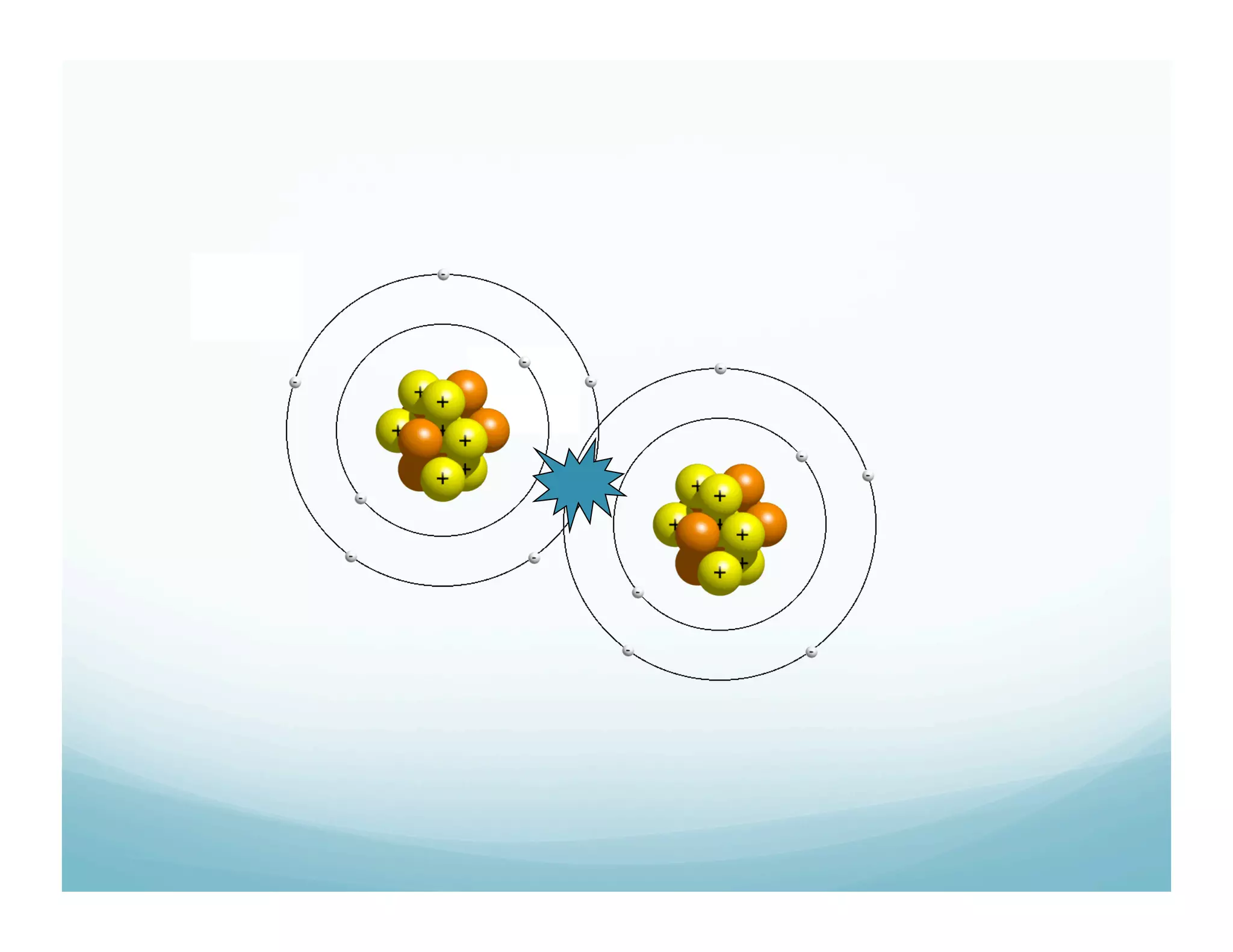
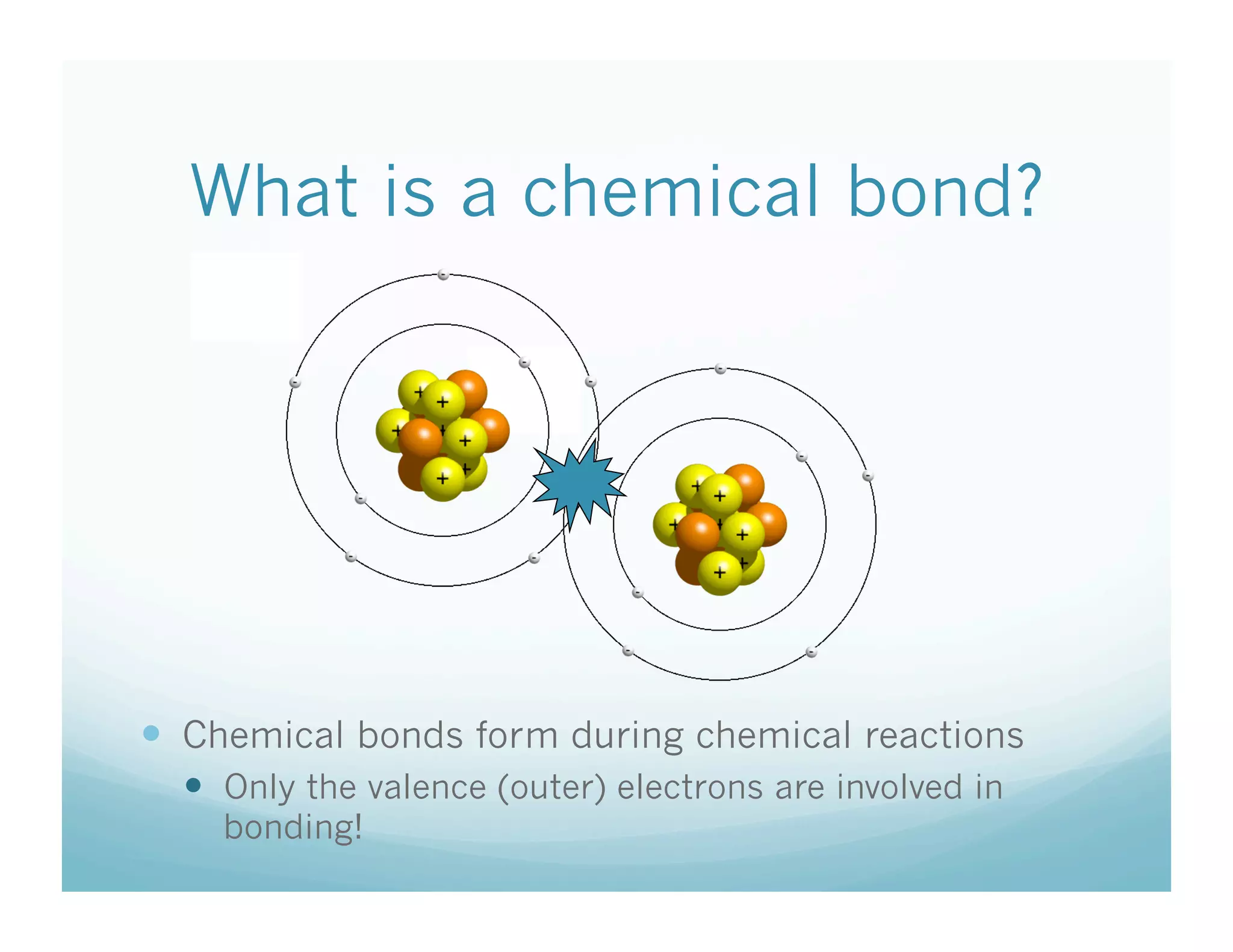
This document summarizes a chemistry lesson on chemical bonds. Students are instructed to answer questions about why unstable isotopes decay and how nuclear reactions differ from chemical reactions. The lesson defines chemical bonds as holding atoms together to form compounds and molecules, involving only valence electrons. It explains that atoms form bonds to achieve stable electron configurations like noble gases, following the octet rule of having 8 valence electrons.