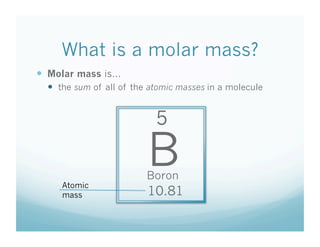
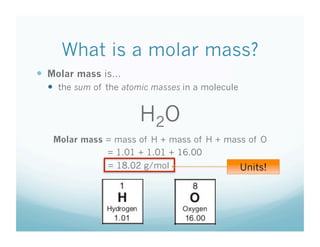
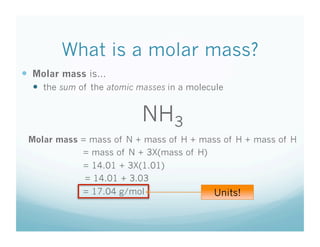
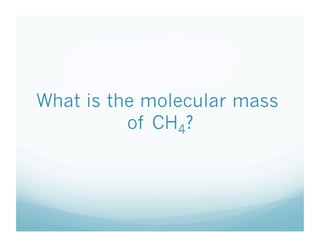
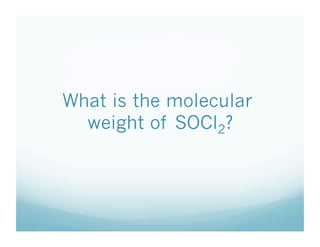
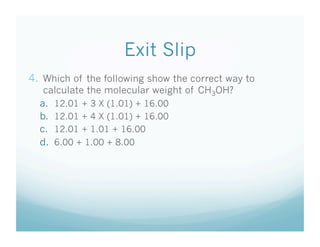
This document contains a chemistry lesson on molar mass. It defines molar mass as the sum of the atomic masses in a molecule. It provides examples of calculating the molar mass of different compounds by adding up the atomic masses of each element. The lesson concludes by having students practice calculating molar masses in groups and answering an exit slip with questions.