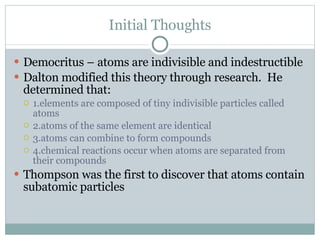
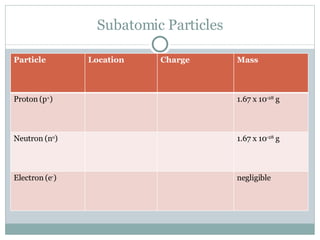
The document discusses the structure of atoms. It defines an atom as the smallest particle of an element that retains its identity in chemical reactions. It describes how atoms are composed of subatomic particles including protons, neutrons, and electrons. It discusses how the number of protons determines the element, the number of electrons usually equals the number of protons, and the mass number is the total of protons and neutrons. Isotopes are defined as atoms of the same element with different numbers of neutrons. The document also discusses electron shells and valence electrons.