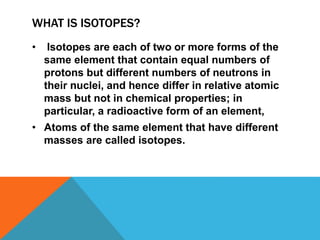
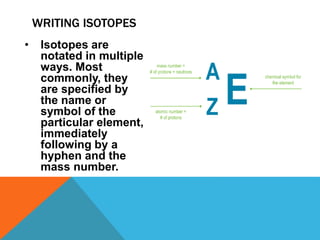
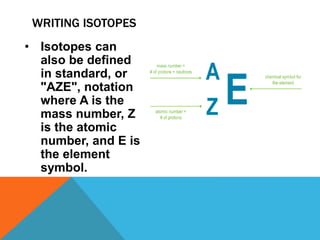
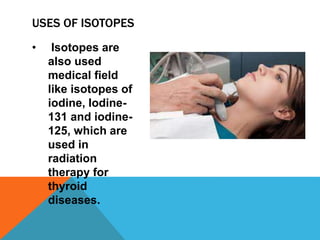
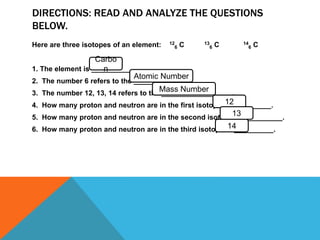
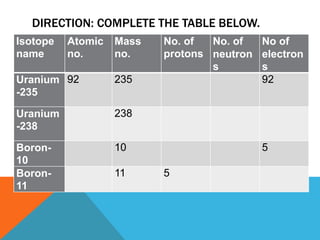
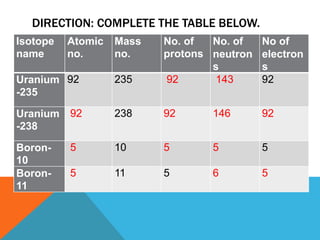
This document discusses isotopes and their properties. It defines isotopes as forms of the same element that have different numbers of neutrons but the same number of protons. It provides examples of carbon isotopes and explains that isotope notation specifies the element name or symbol followed by the mass number. Isotopes can be naturally occurring or artificially made, and they have various medical and dating uses. The document also includes questions to test the reader's understanding of isotopes.