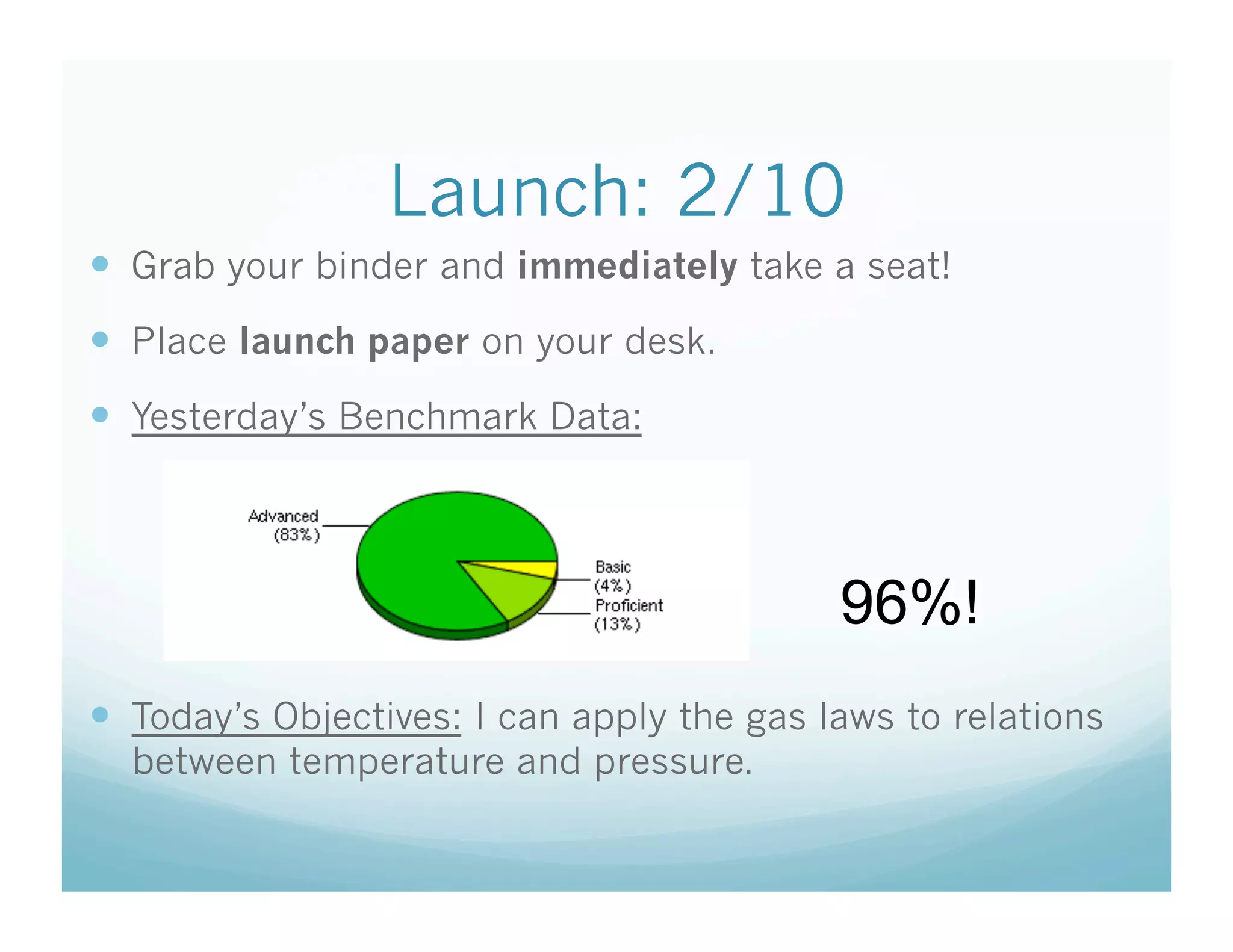
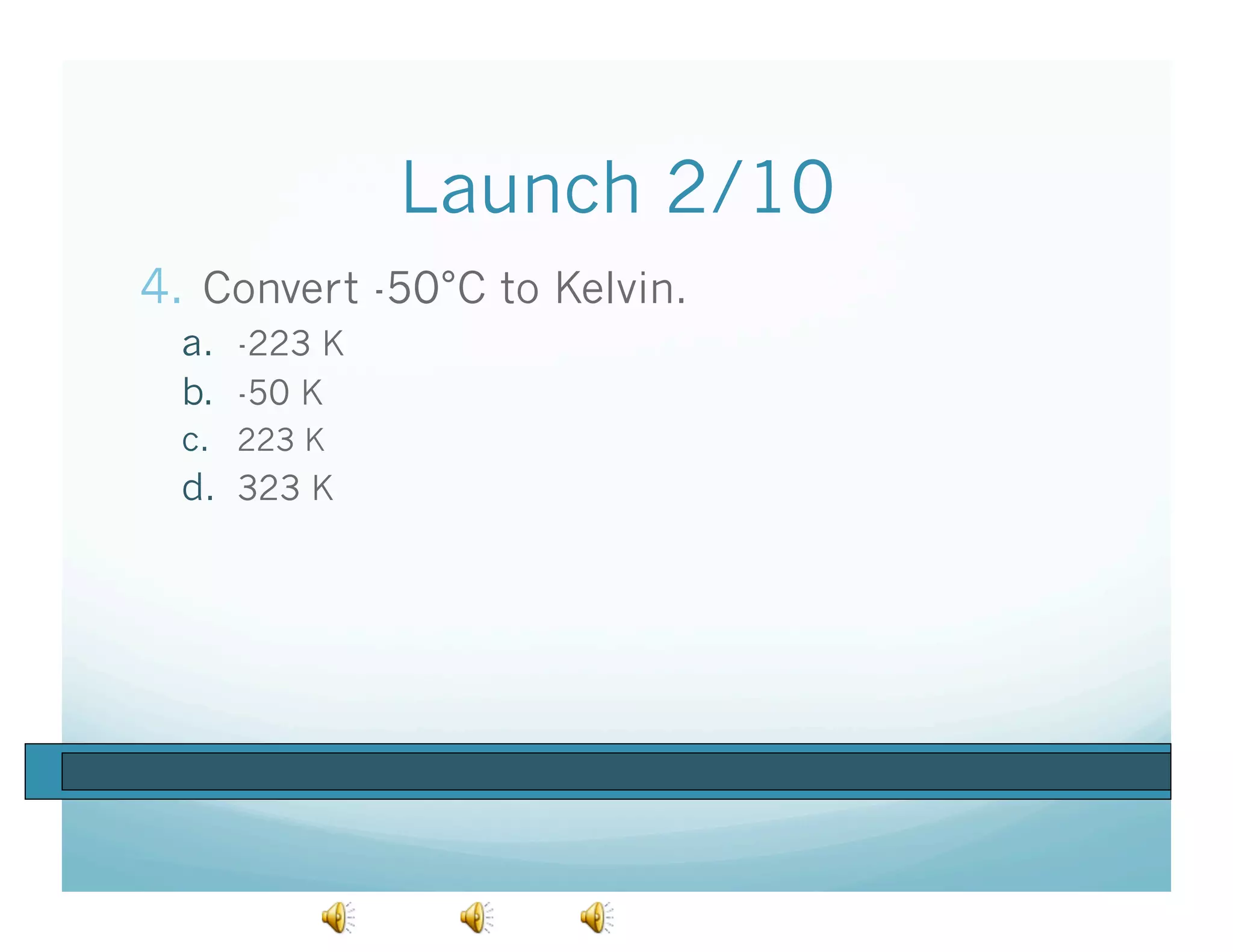
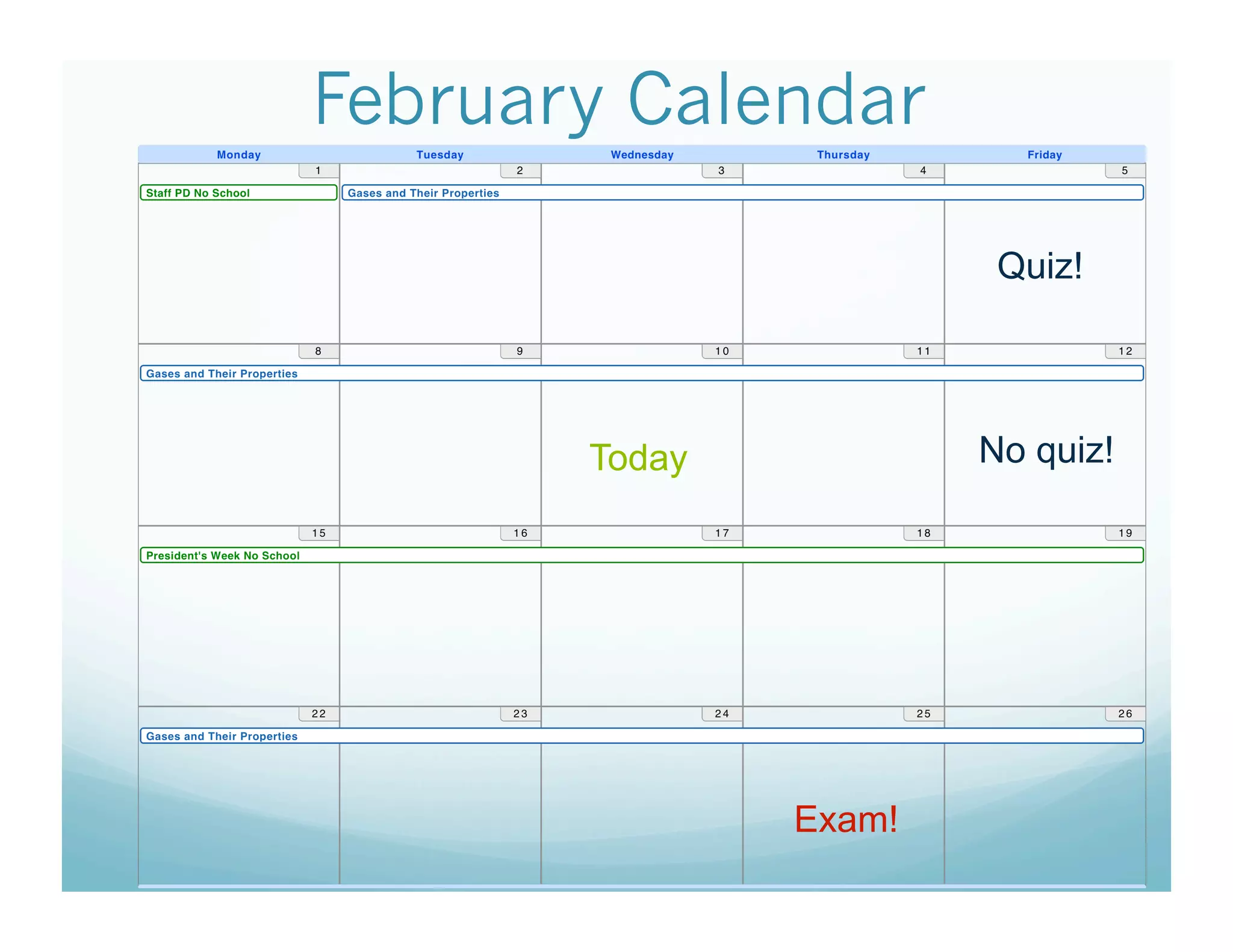
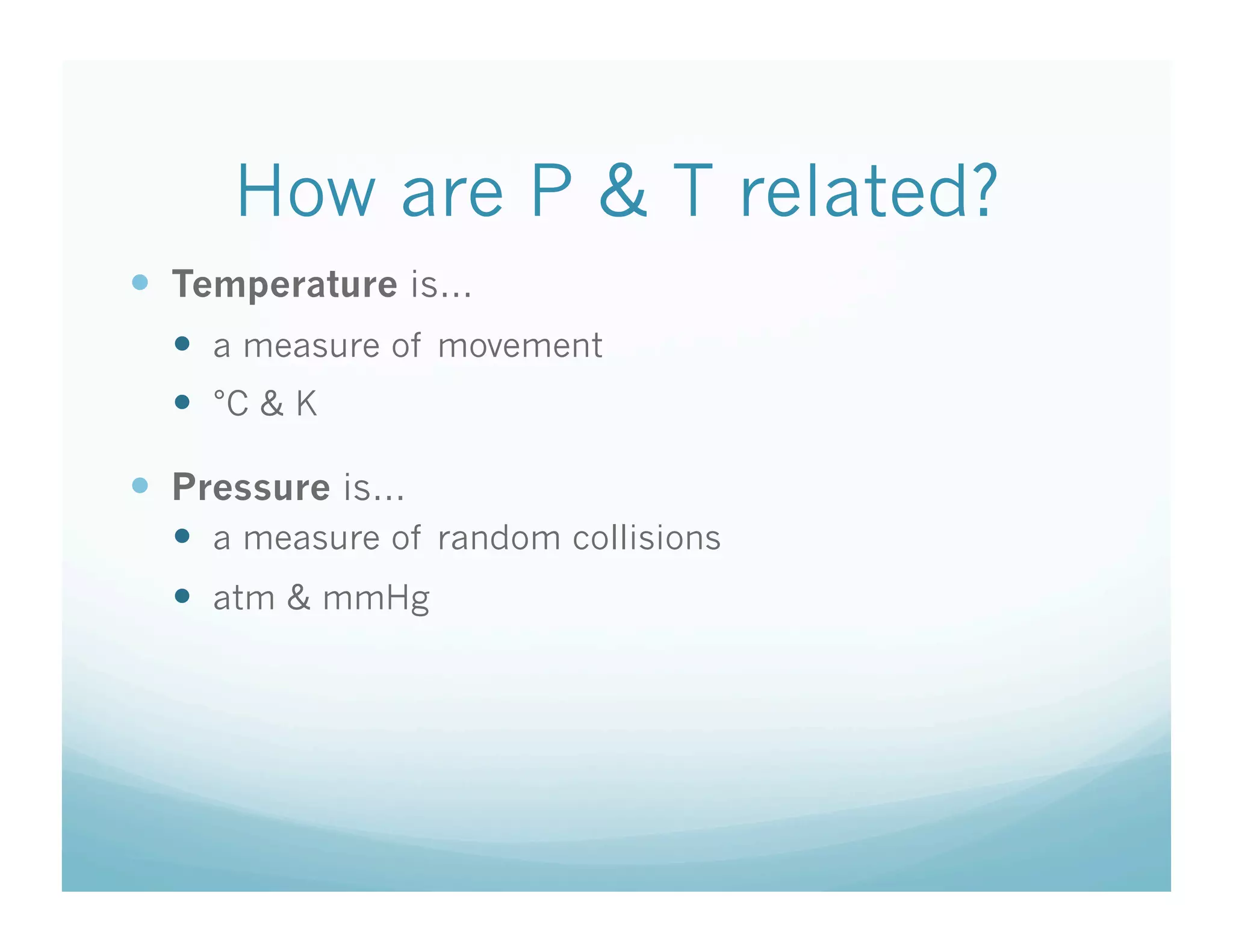
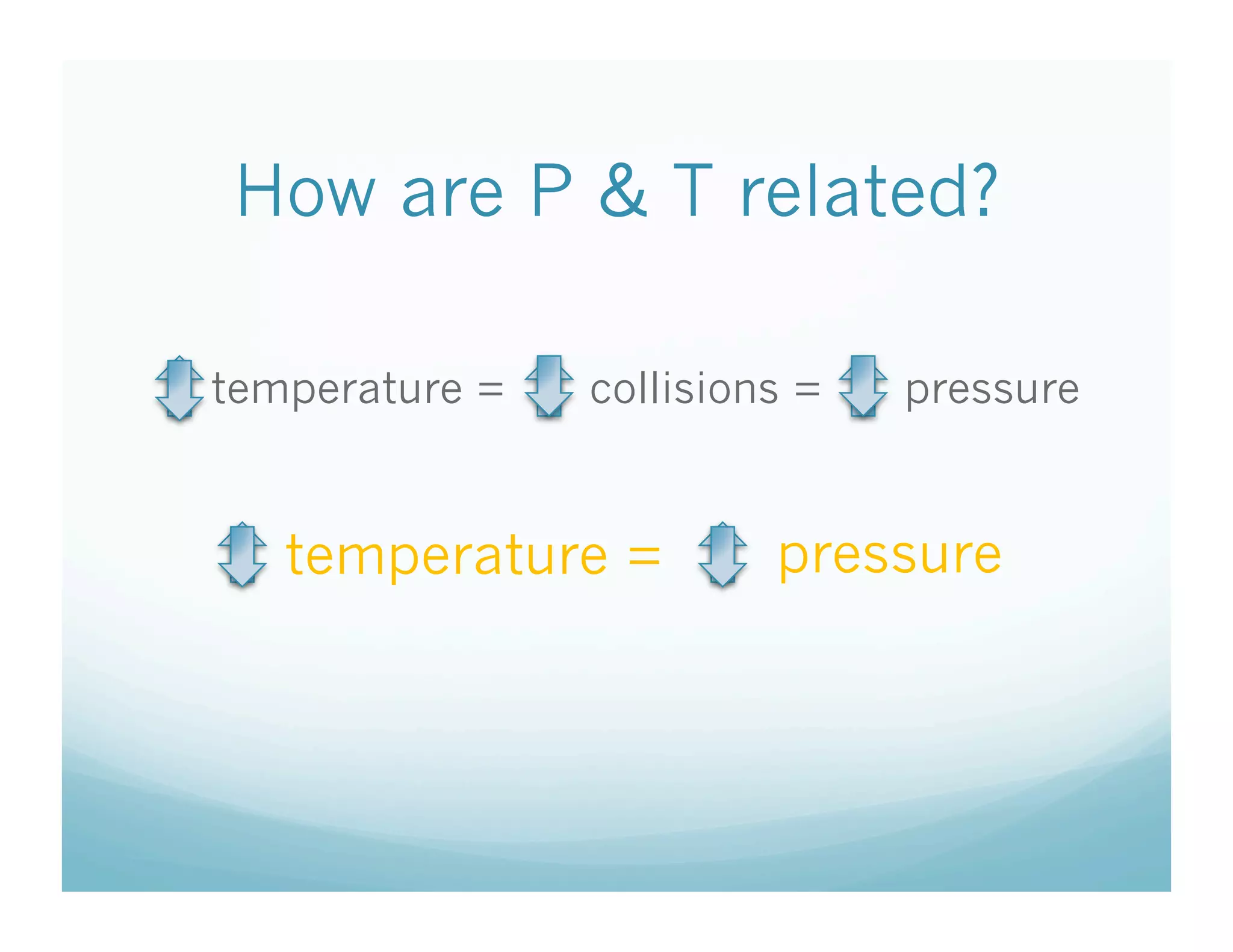
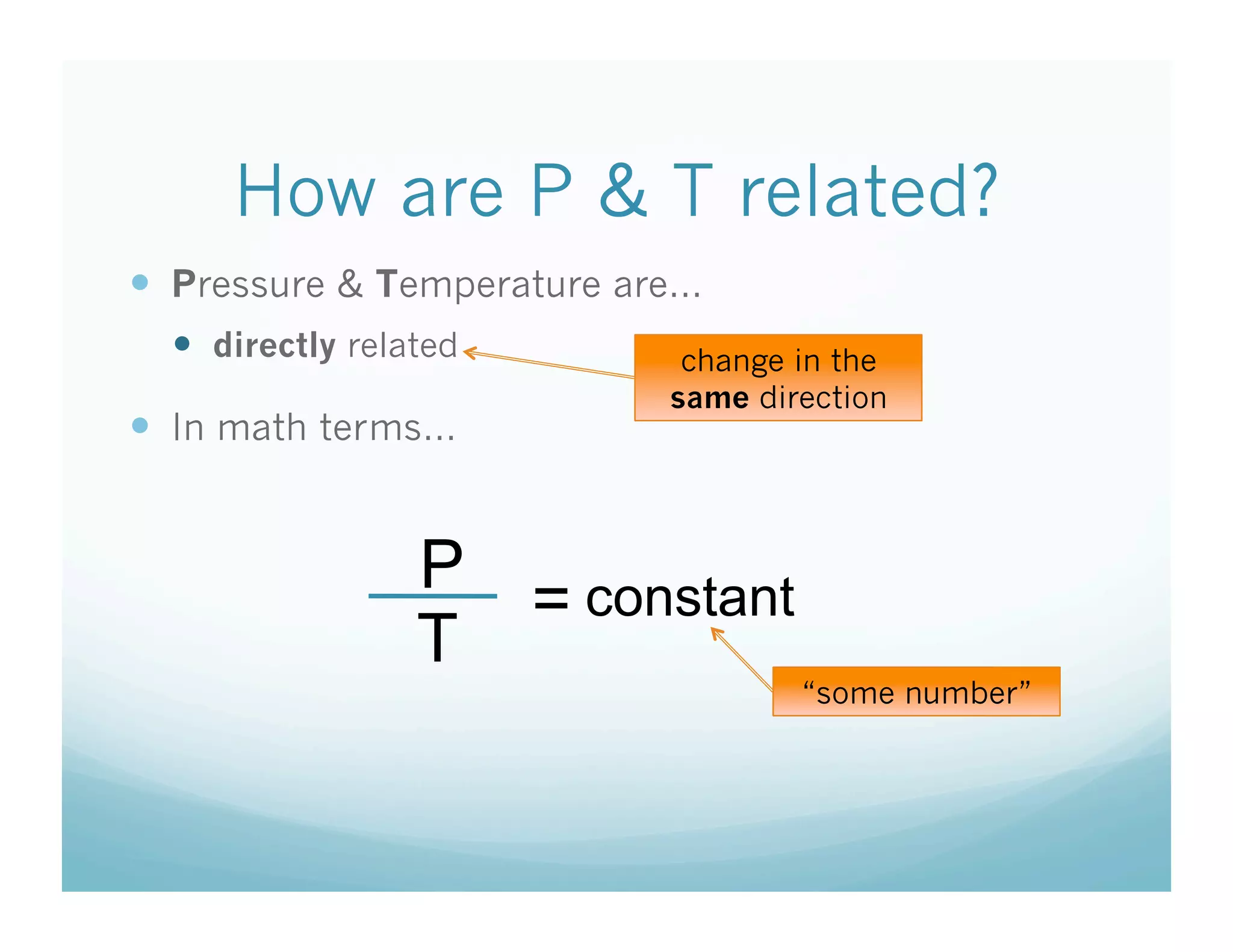
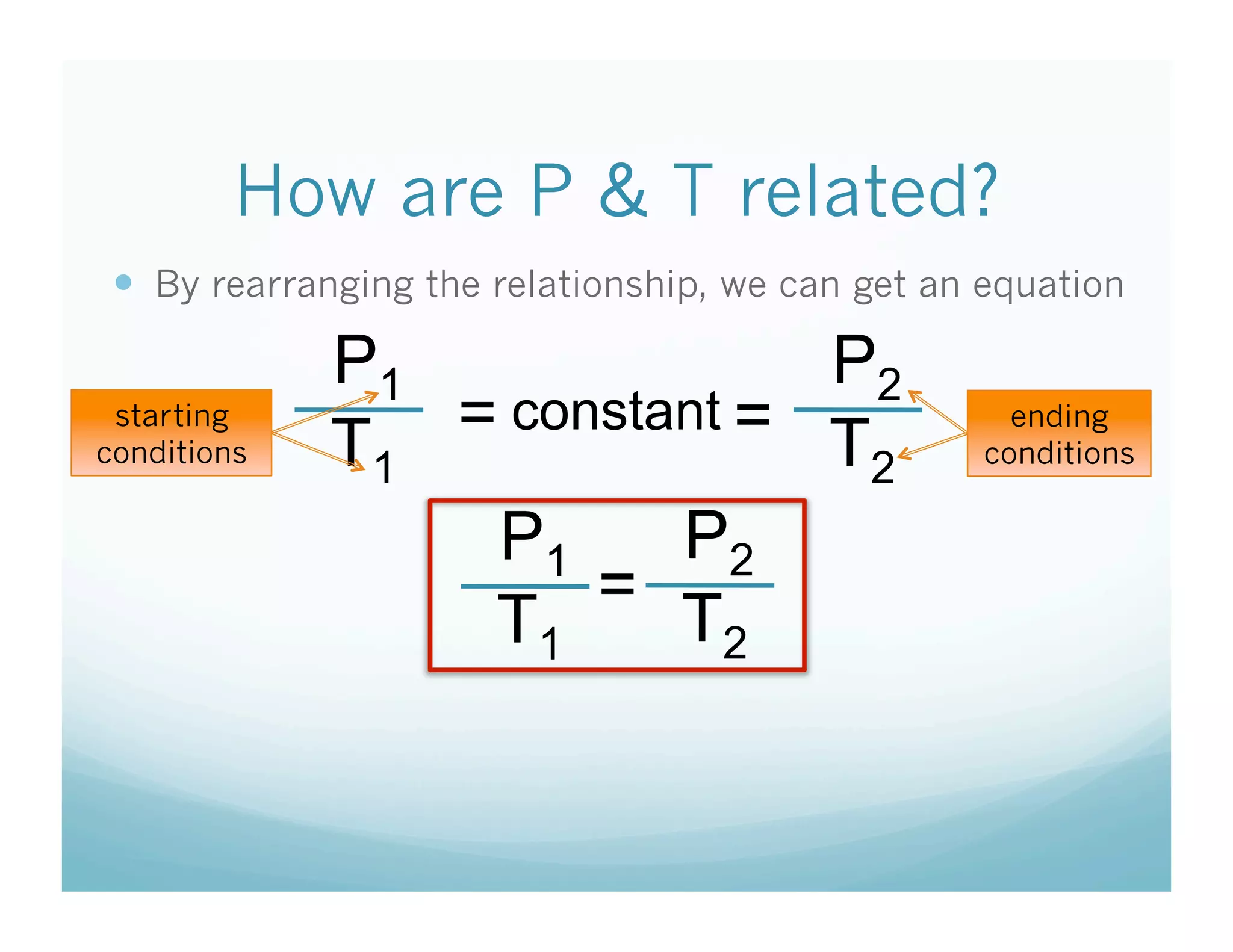
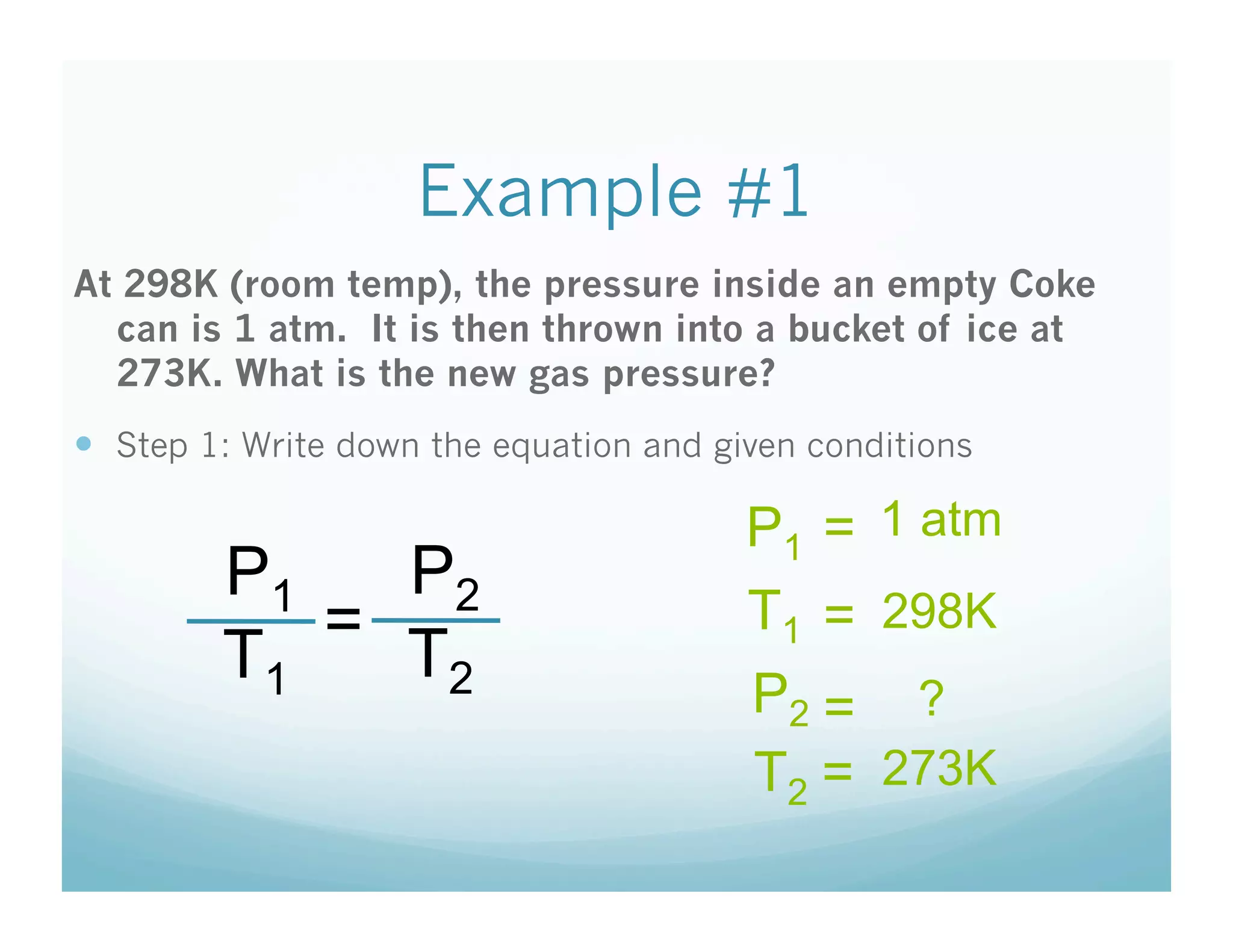
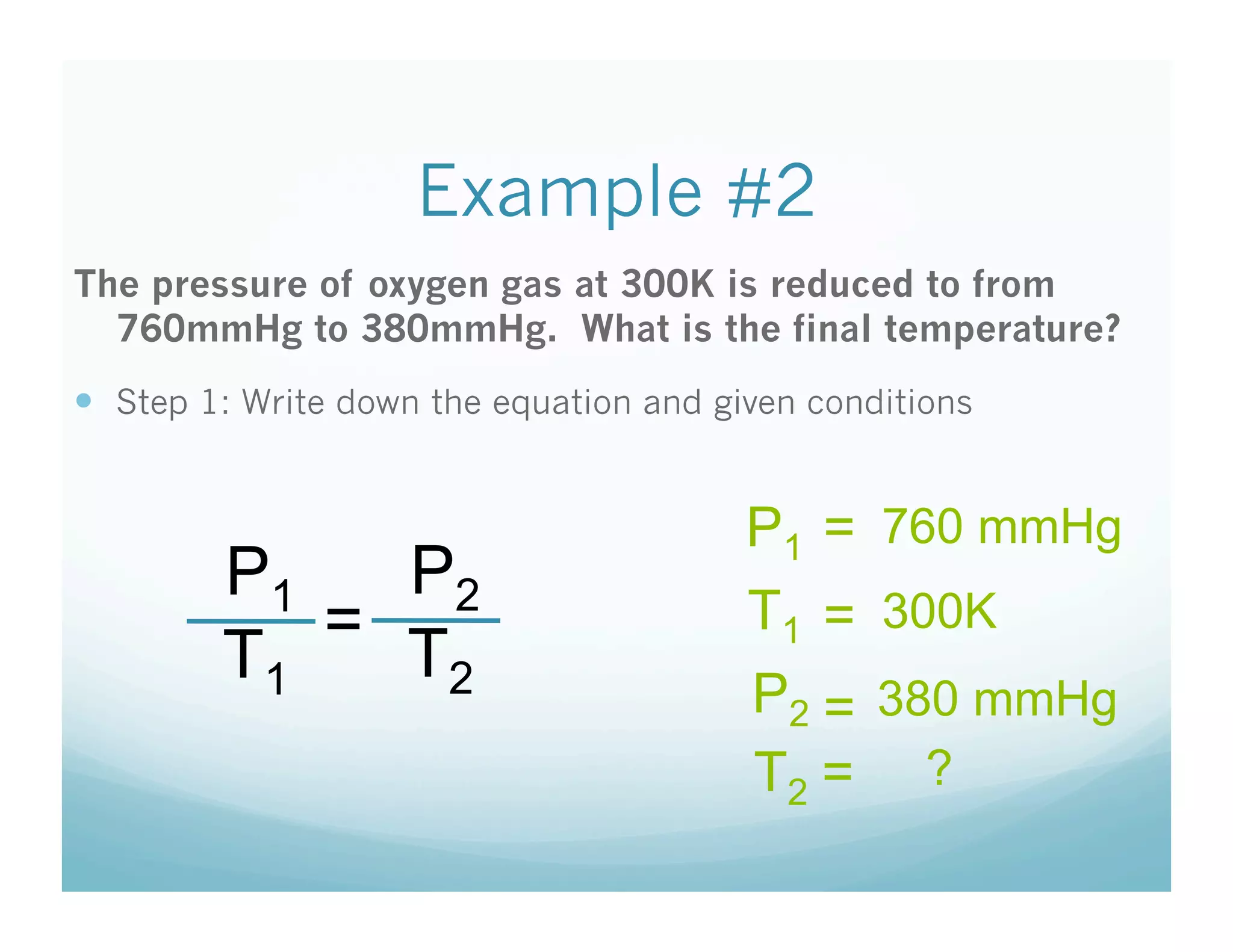
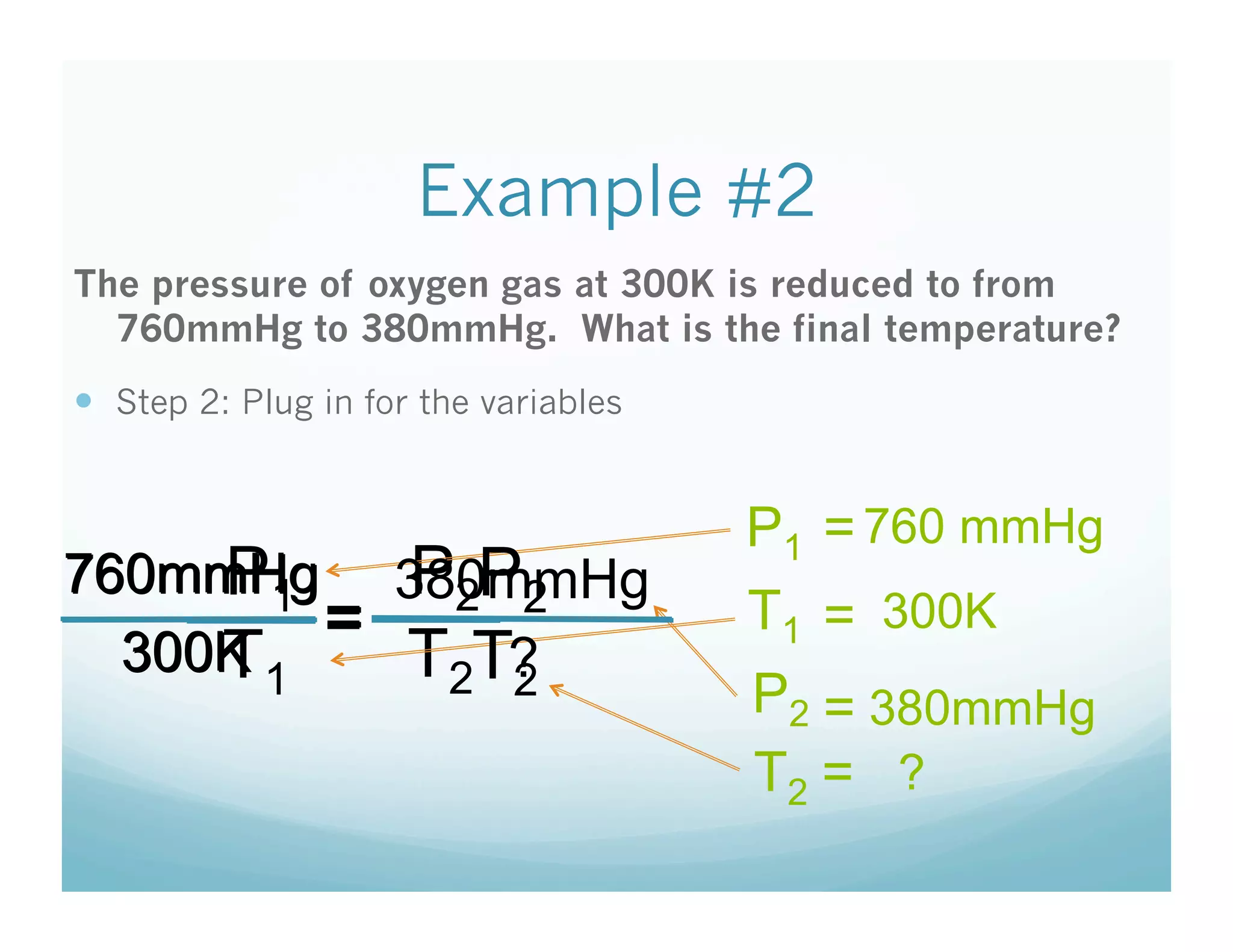
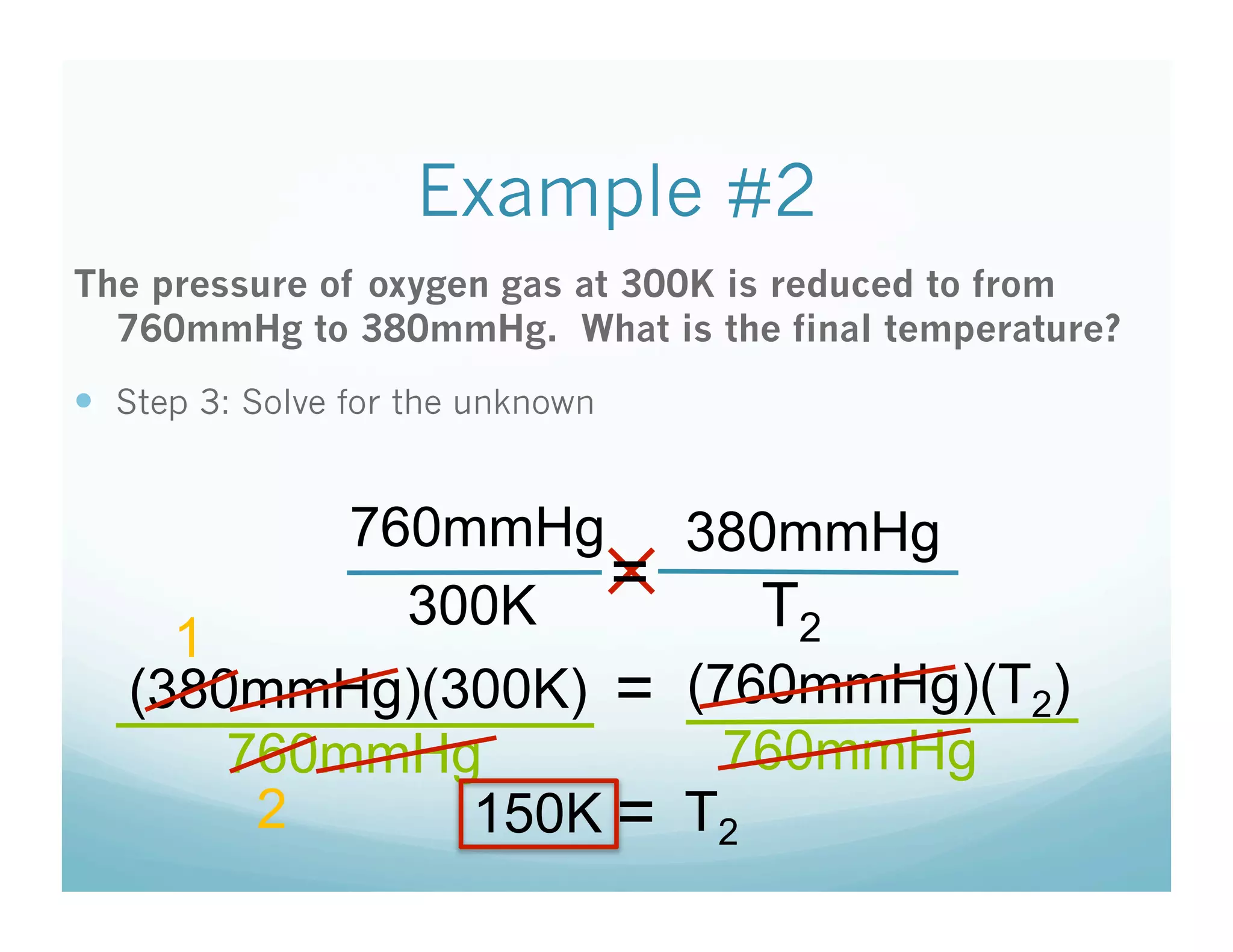
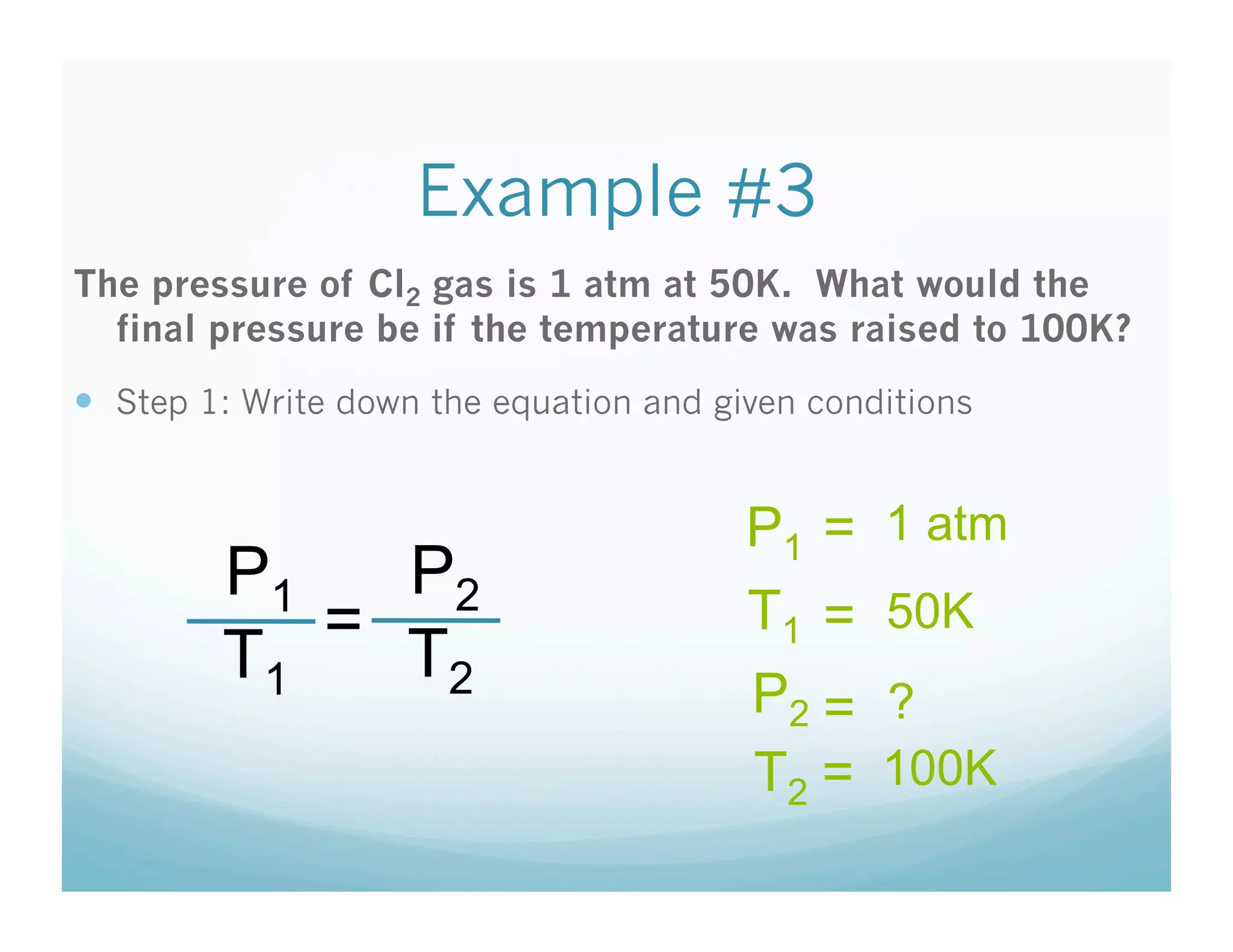
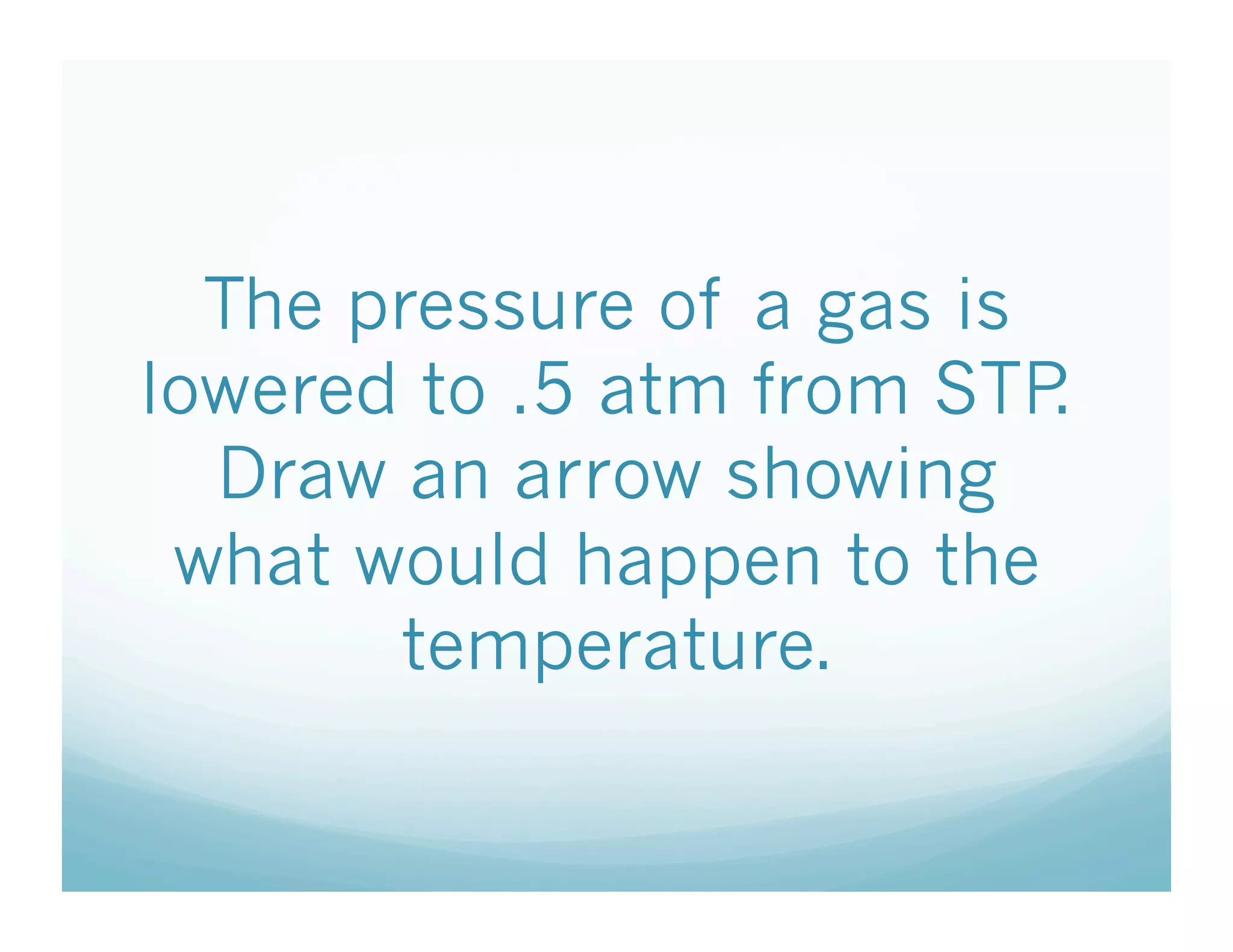
The document provides information about a chemistry lesson on gas laws and the relationship between temperature and pressure. It includes objectives, examples, and questions for students. The lesson explains that temperature and pressure are directly related, as temperature increases so does molecular motion and collisions, thus increasing pressure. Examples show how to use the direct relationship equation to calculate new pressure or temperature given one variable.