Unit 1 lesson 12ppt
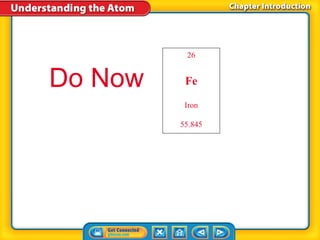
- 1. 26 Do Now Fe Iron 55.845
- 2. Do Now 26 Fe Average atomic mass 1.The ____________________________ is a weighted Iron average that reflects the abundance of different 55.845 isotopes. Mass number 2.The ______________________________ is determined by adding the number of protons and neutrons in an atom. Isotope 3.__________________________ are atoms of the same element that have different numbers of neutrons. Atomic Number 4.An element’s ____________________________ and the number of protons in the nucleus of its atoms are always the same.
- 3. Different Elements—Different Numbers of Protons the number of protons The type of the number of electrons element
- 4. What are the atomic numbers of carbon, nitrogen and oxygen? Carbon = 6, Nitrogen = 7, Oxygen = 8 Explain the difference between an oxygen atom and a carbon atom. Oxygen has 8 protons. Carbon has 6 protons.
- 5. What are two numbers the can be used to identify an element? The number of protons and electrons Explain the difference between a Boron atom (B) and an Oxygen atom. Boron has 5 protons and 5 electrons Oxygen has 8 protons and 8 electrons Analyze the periodic table and tell how you think it is organized Organized by increasing atomic #
- 6. 1. Fluorine has an atomic number of 9. What can you conclude about an atom of fluorine A) It has 9 protons A) It has 9 protons
- 7. 2. In what part of an atom can protons be found? C) Inside the atomic nucleus C) Inside the atomic nucleus
- 8. 3. Since atoms are very small, what can you infer about atomic mass units? D) Atomic mass units and grams D) Atomic mass units and grams Measure different properties Measure different properties
- 9. 4. An atom of flourine has an atomic mass of 19u, Keeping in mind that its atomic number is 9, what can you infer about this atom C) It has ten neutrons C) It has ten neutrons
- 10. 5. If a sulfur atom has 16 protons, 16 electrons, and 16 neutrons, it’s atomic mass is? B) 32 B) 32
- 11. 6. If a hydrogen atom has 1 proton, 1 electron, and 1 neutron it’s atomic number is? A) 1 A) 1
- 12. 7. On the periodic table, how is the atomic mass represented? A) As an average of the mass of A) As an average of the mass of different isotopes different isotopes
- 13. 8. What do carbon-12 and Carbon-14 have in common? A) They have the same number of A) They have the same number of protons protons
- 14. 9. How is Carbon-12 different from Carbon-14? C) They have a different number of C) They have a different number of neutrons neutrons
- 15. 10.What can you conclude about Carbon-14 from its name? D D
- 16. Neutrons and Isotopes • Atoms of the same element can have different numbers of neutrons. • Isotopes are atoms of the same element that have different numbers of neutrons. • Most elements have several isotopes.
- 17. Why do the isotopes of carbon in the table have different mass numbers? They have a different number of neutrons
- 18. Ions—Gaining or Losing Electrons • An ion is an atom that is no longer neutral because it has gained or lost electrons. • An ion can be positively or negatively charged depending on whether it has lost or gained electrons.
- 19. • When a neutral atom loses one or more electrons, it has more protons than electrons and as a result, has a positive charge. • An atom with a positive charge is called a positive ion.
- 20. • When a neutral atom gains one or more electrons, it now has more electrons than protons and as a result, has a negative charge. • An atom with a negative charge is called a negative ion.
- 21. neutral An ion is an atom that is no longer ________________ because it has gained lost ______________________ or _____________________ electrons. An atom that lost An atom that gained an electron an electron Positive Charge Negative Charge
- 22. Ions—Gaining or Losing Electrons (cont.) How does a neutral atom change when its number of protons or electrons changes?