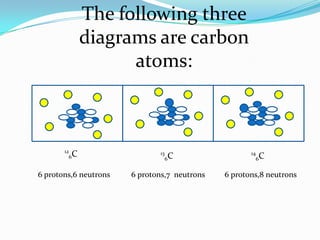
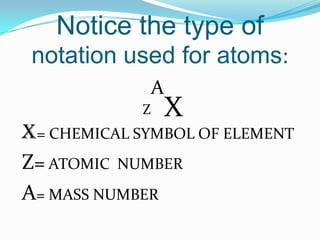
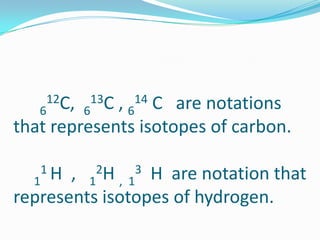
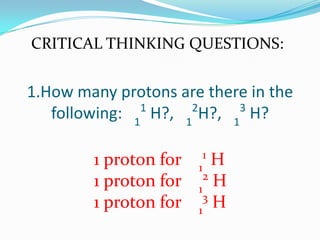
The document provides information about the structure of atoms, including:
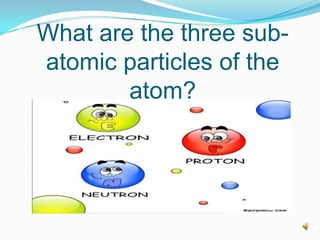
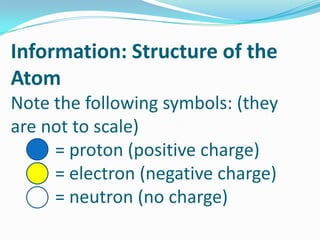
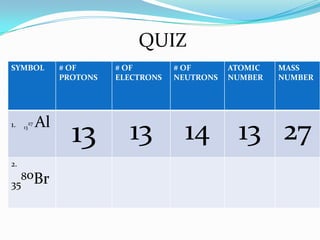
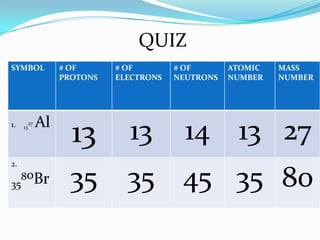
1) The three main subatomic particles that make up atoms are protons, neutrons, and electrons.
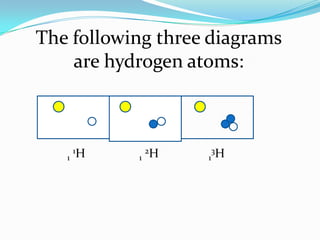
2) Isotopes are atoms of the same element that differ in their number of neutrons.
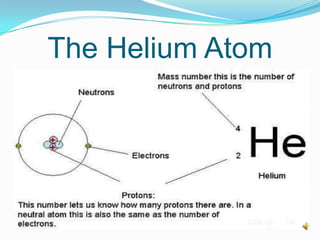
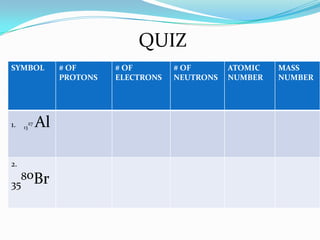
3) The atomic number identifies the number of protons in an atom, while the mass number provides the total number of protons and neutrons.