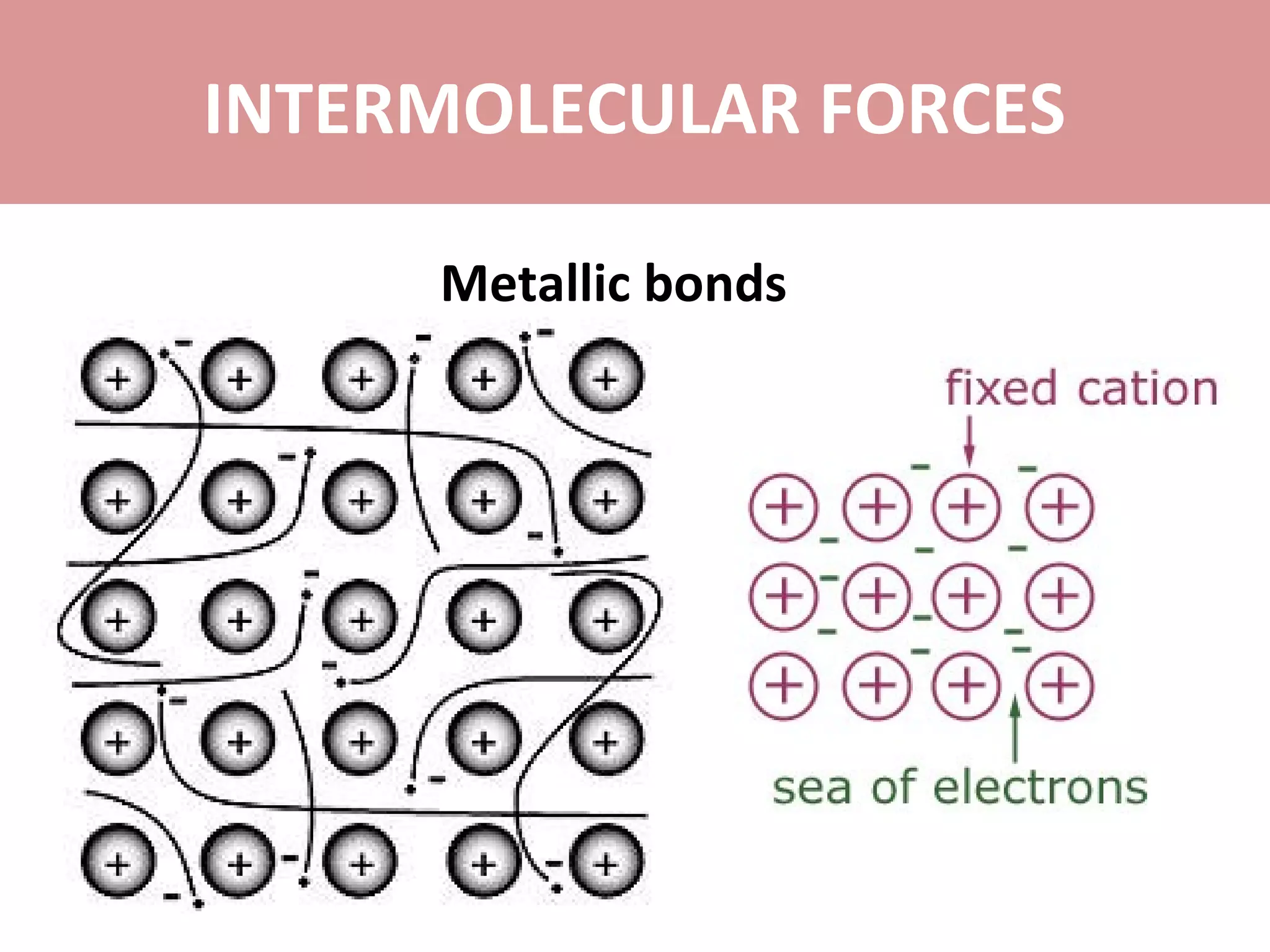
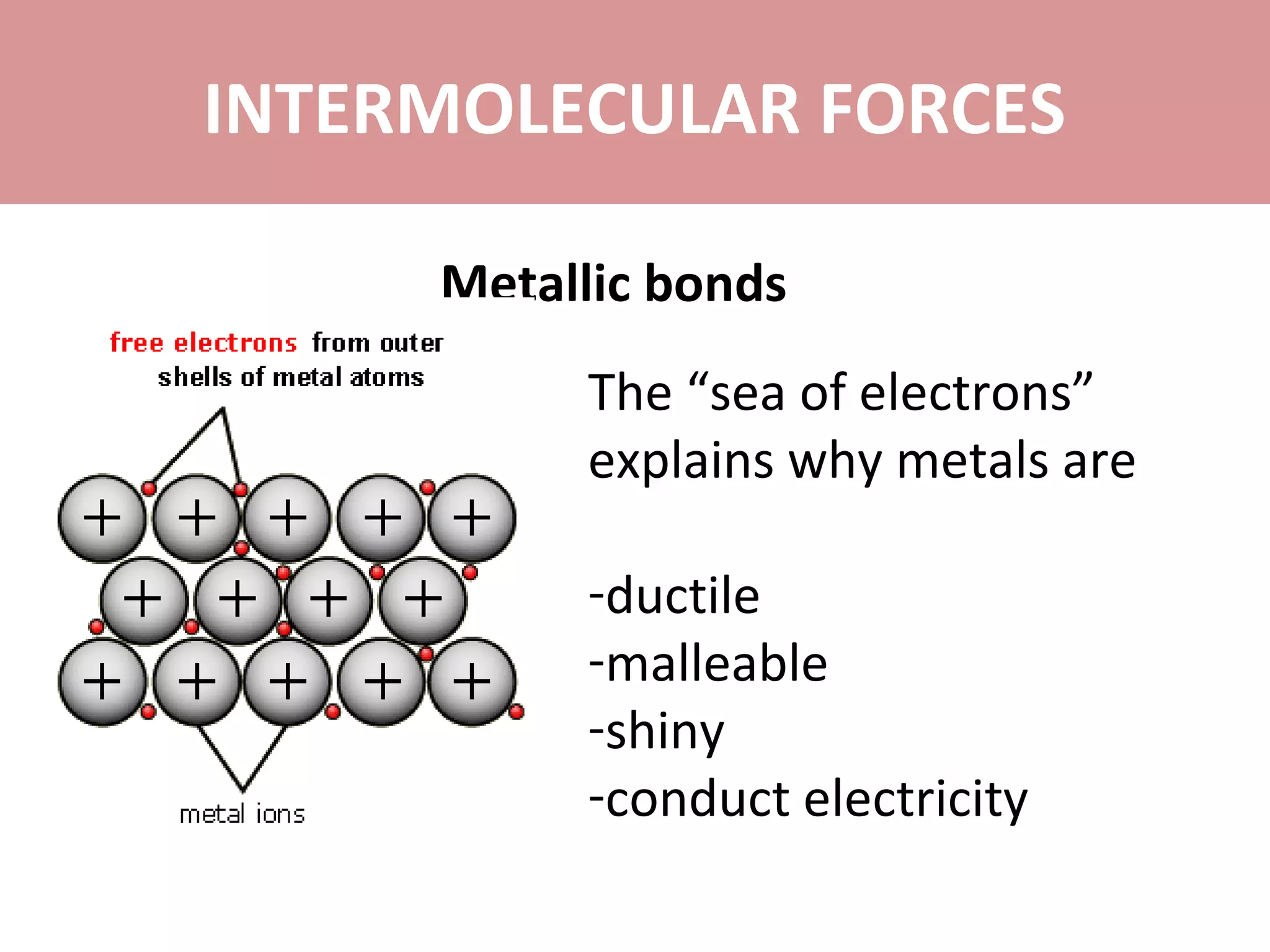
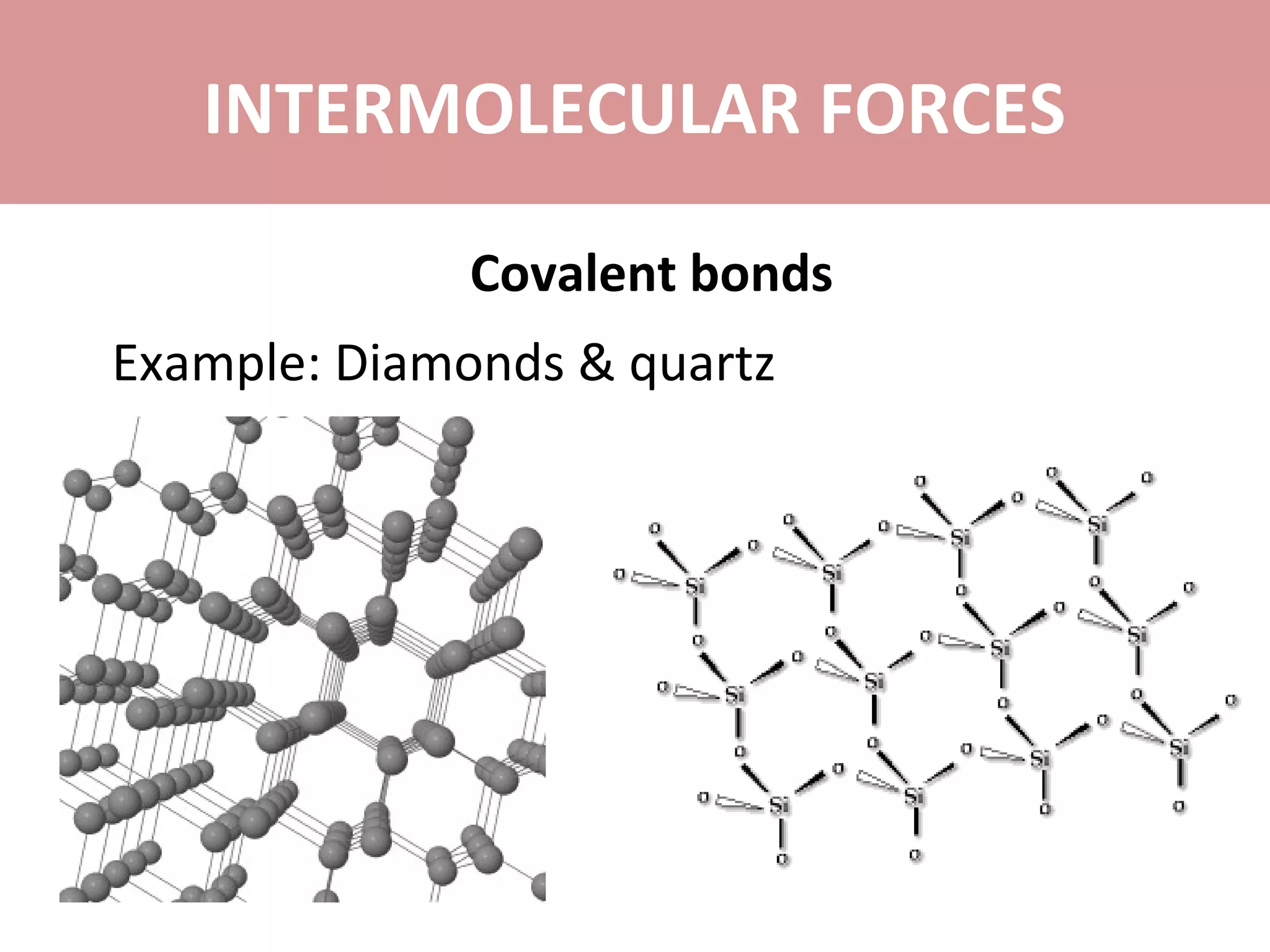
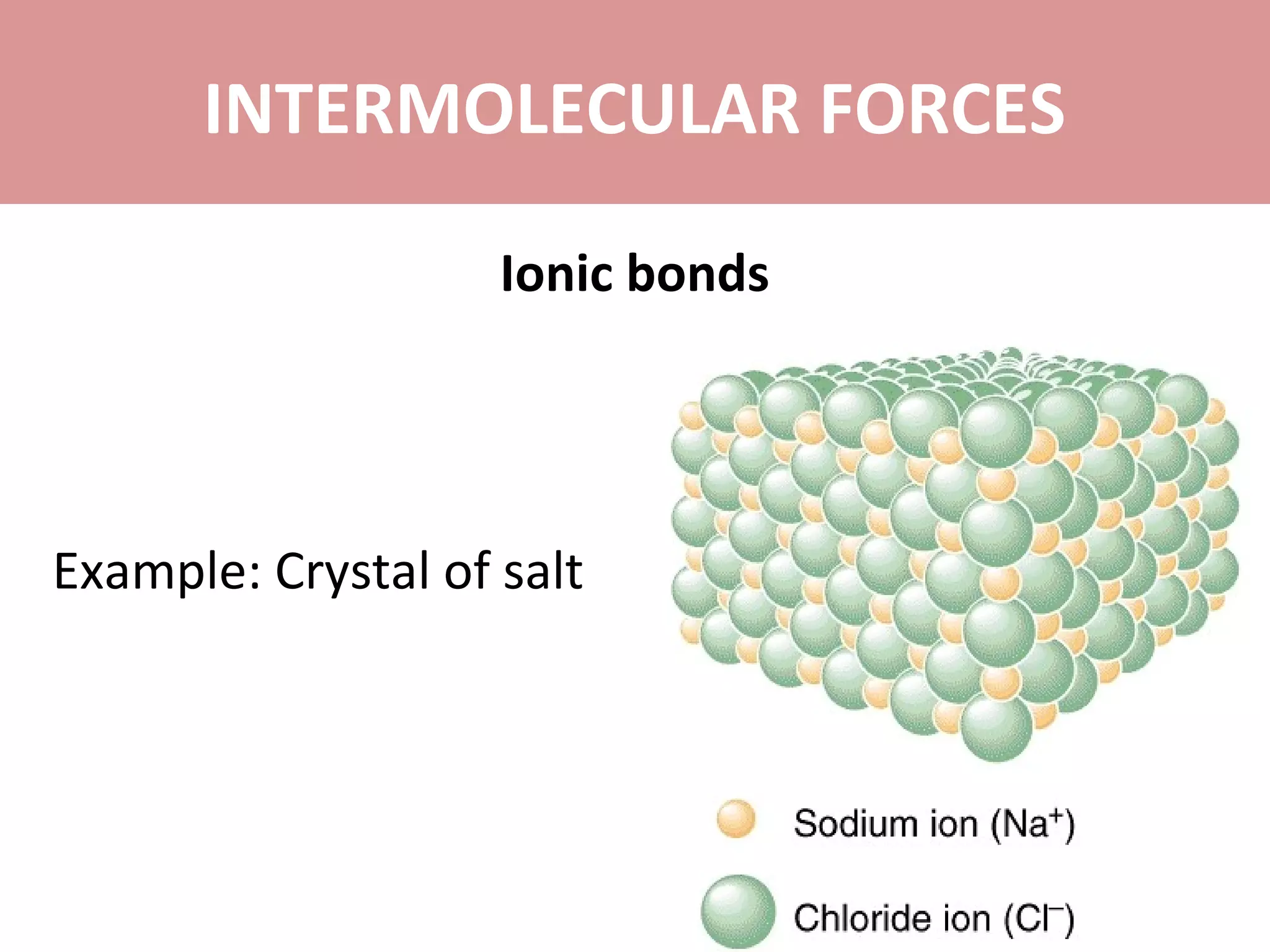
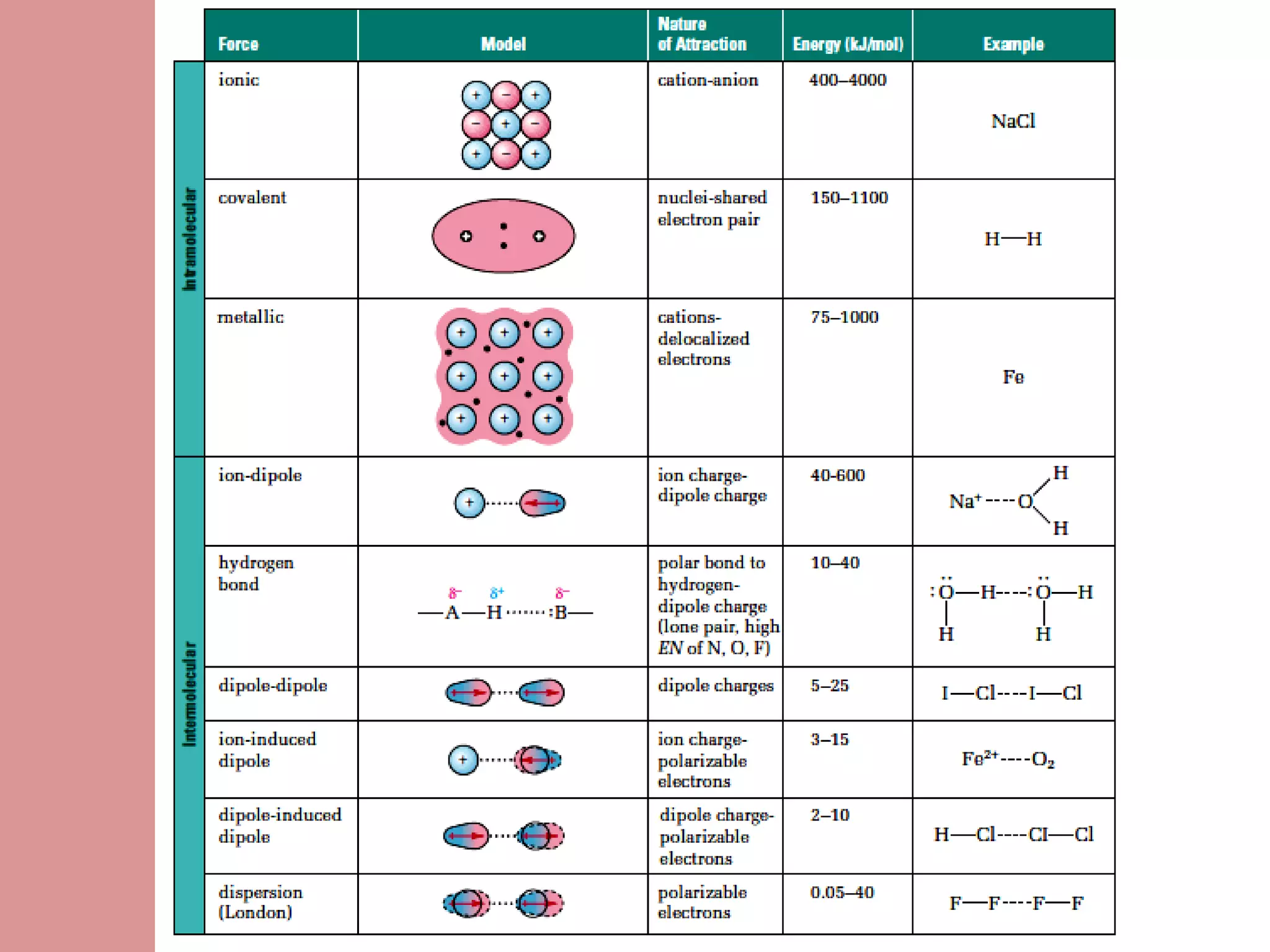
The document discusses the different types of intermolecular forces:
1) London dispersion forces involve temporary dipoles induced in molecules by fluctuating electron densities.
2) Induced-dipole forces involve a permanent dipole inducing a temporary dipole in a neighboring non-polar molecule.
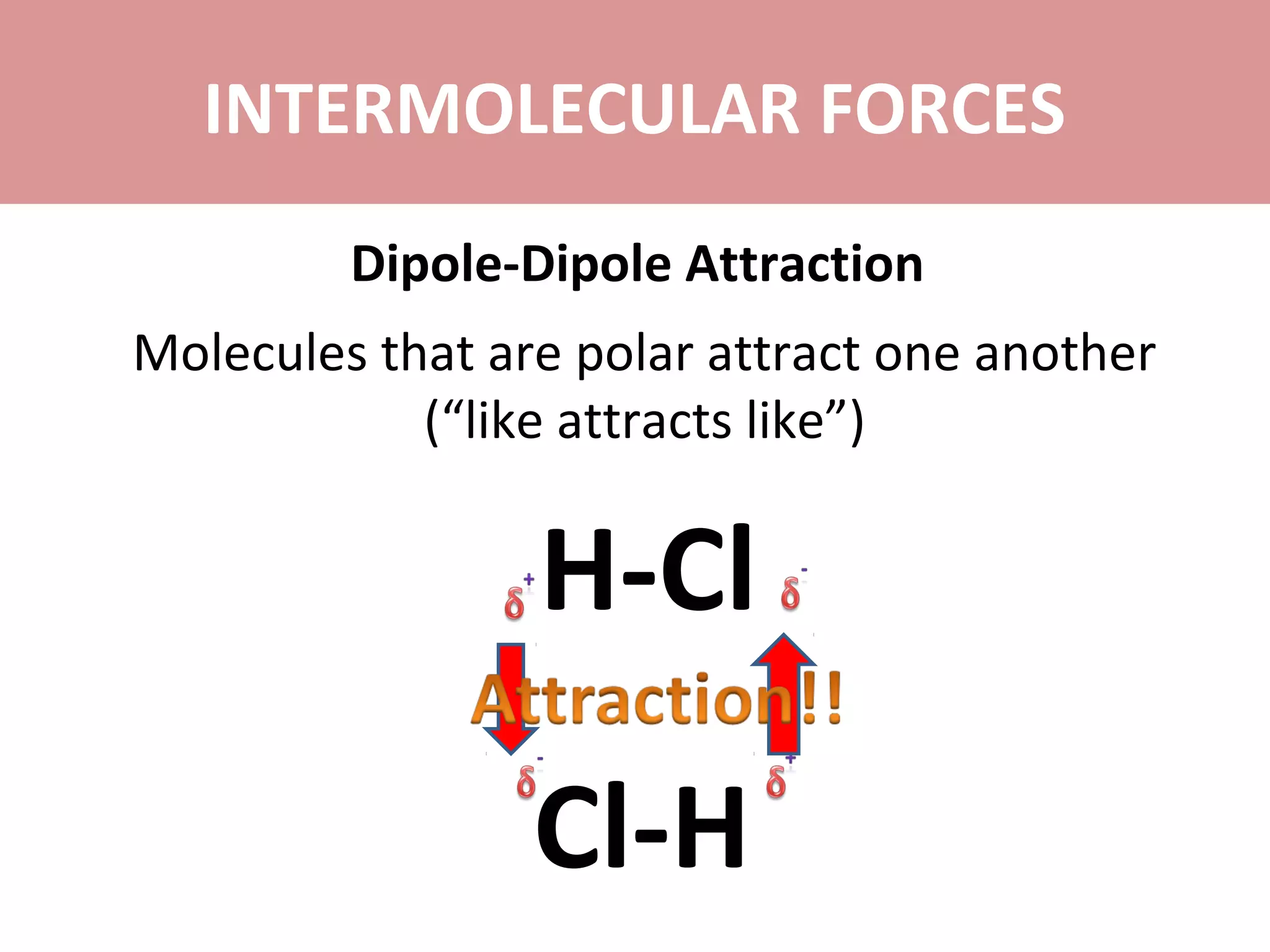
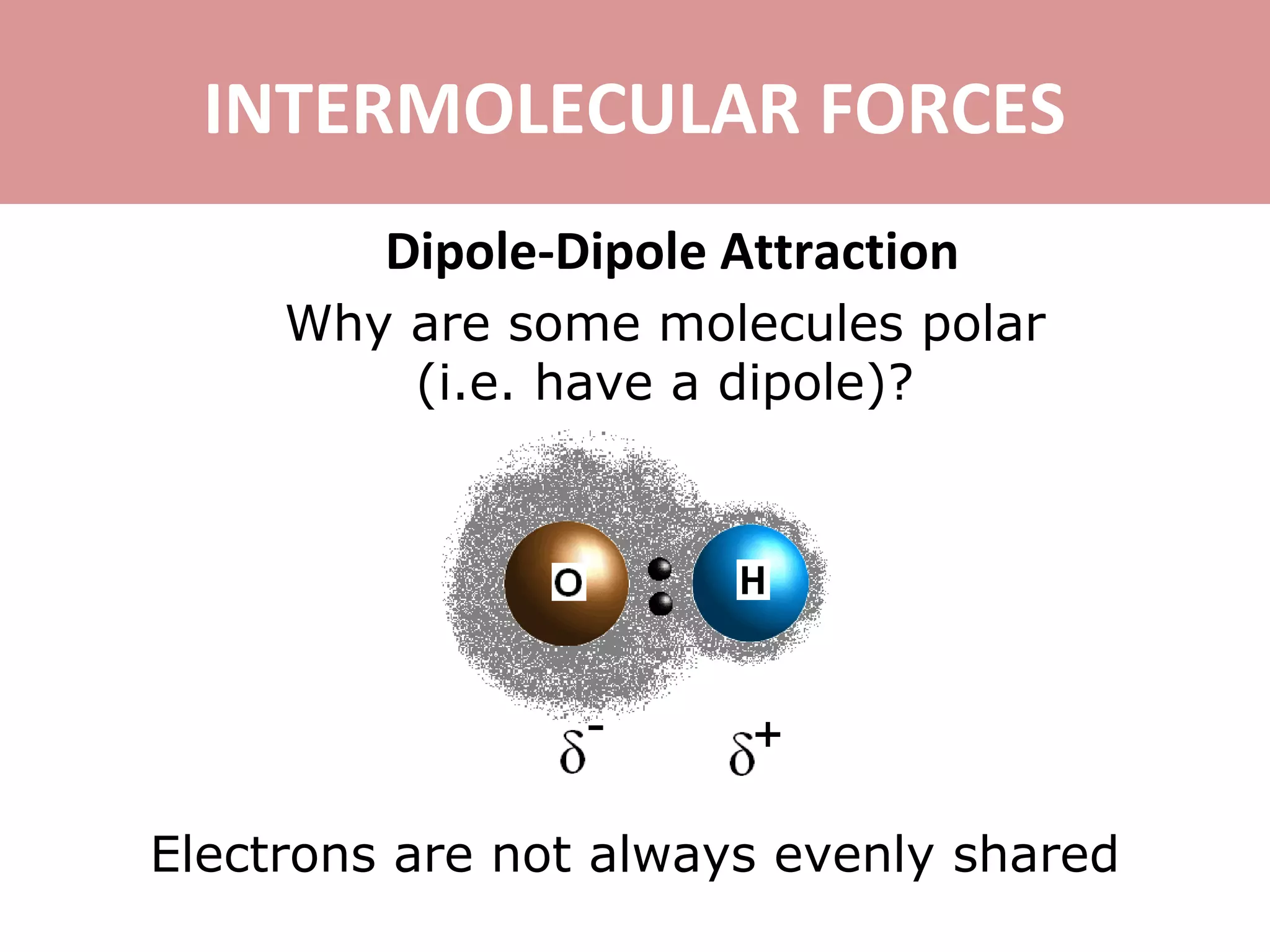
3) Dipole-dipole attraction occurs between polar molecules due to the attraction between their partial charges.
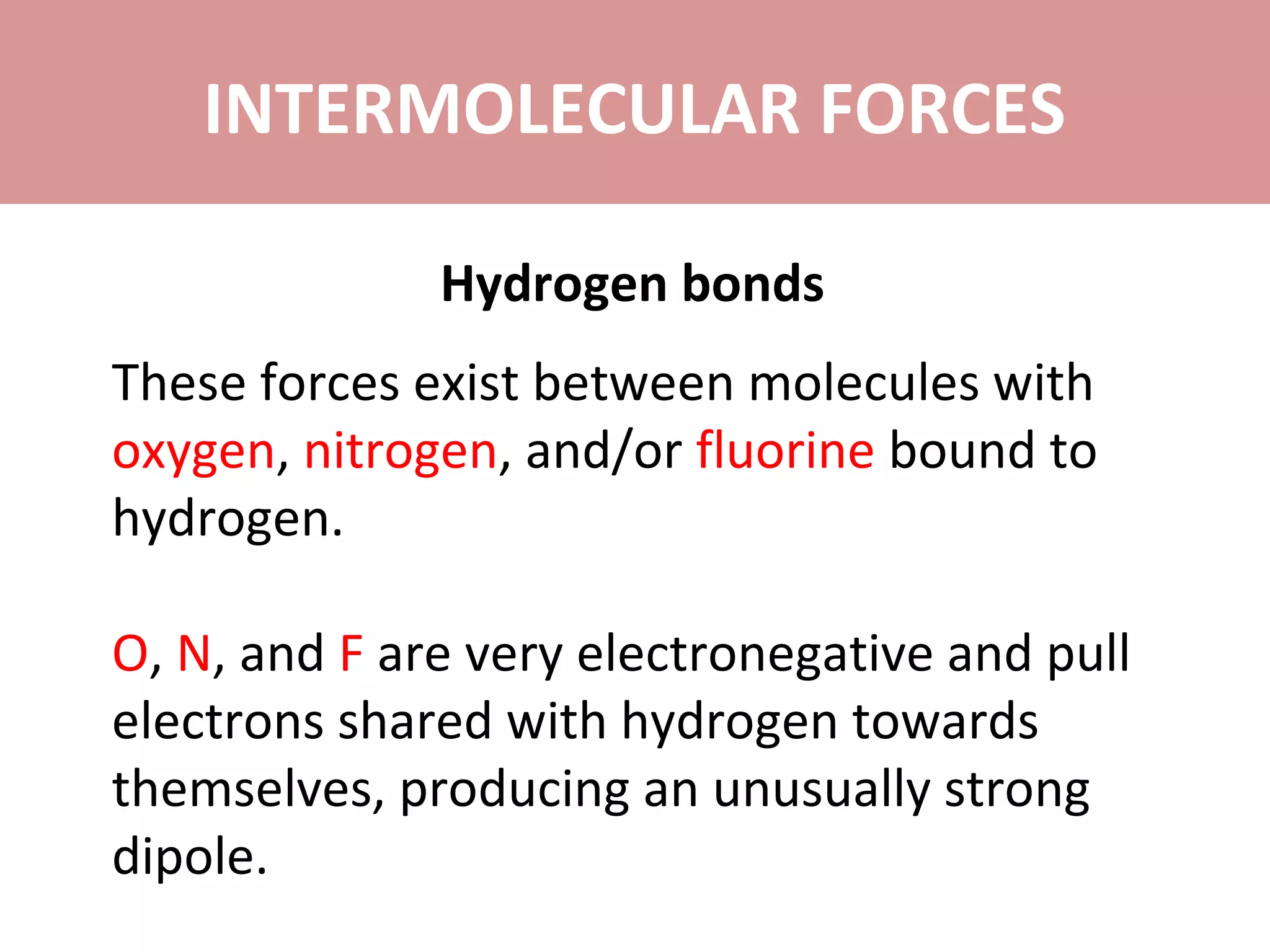
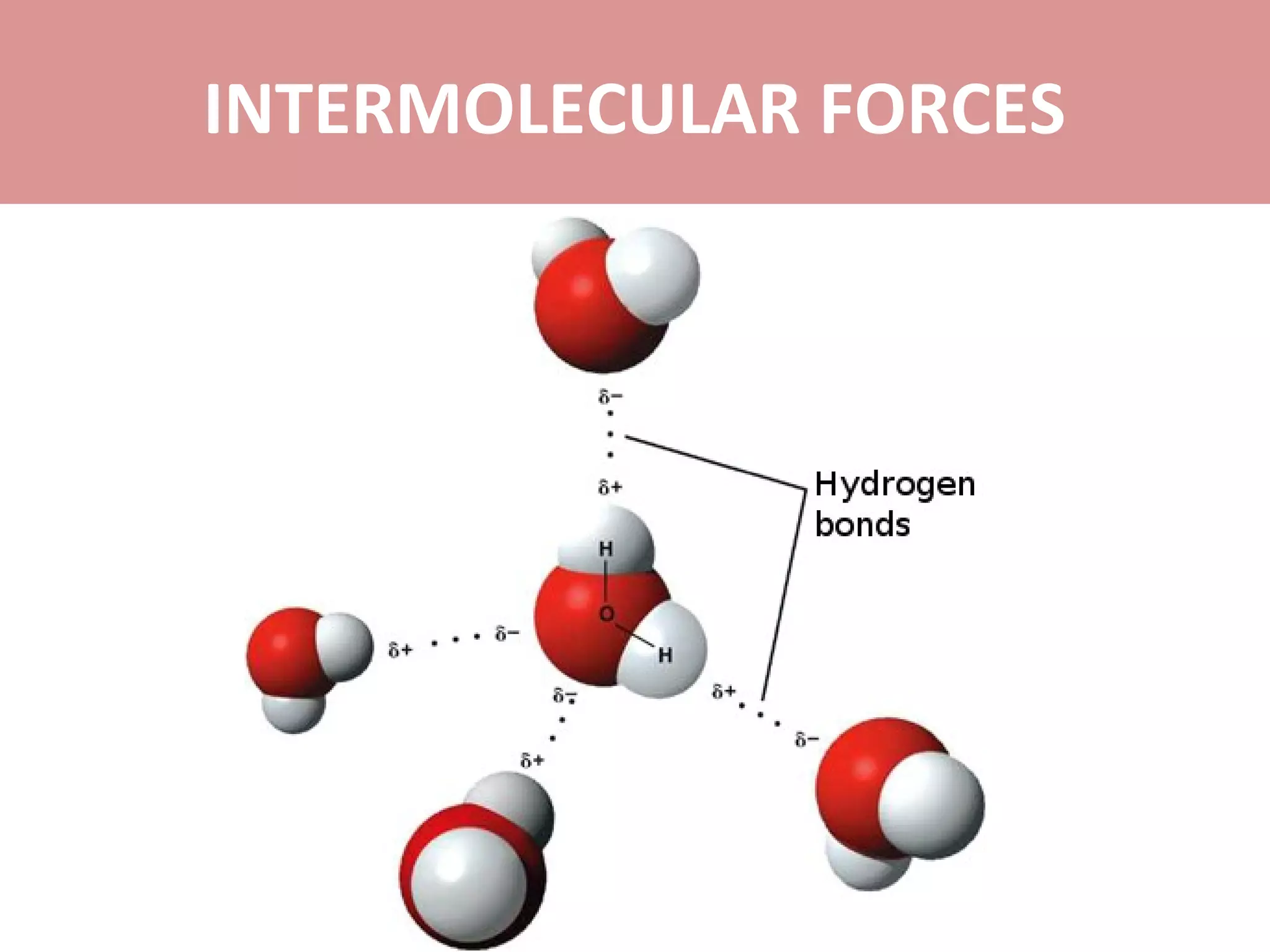
4) Hydrogen bonds are very strong dipole-dipole bonds that exist between molecules with oxygen, nitrogen, or fluorine bonded to hydrogen.