Embed presentation
Downloaded 23 times

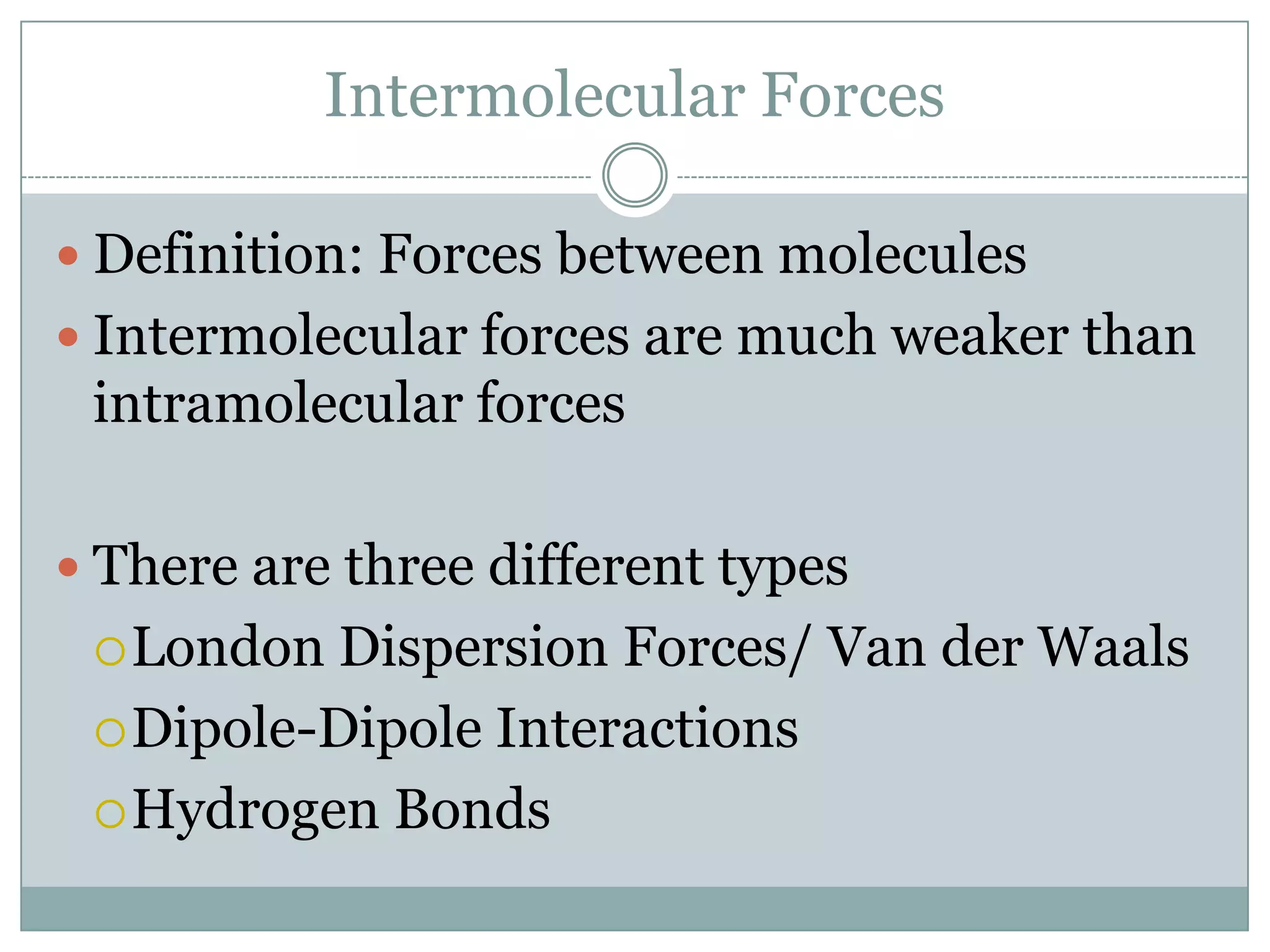




The document discusses intermolecular forces and their effect on solubility. There are three main types of intermolecular forces: London dispersion forces, dipole-dipole interactions, and hydrogen bonding. London dispersion forces are the weakest, while hydrogen bonding is the strongest. Compounds with stronger intermolecular forces have higher melting and boiling points, making them more likely to be liquids or solids rather than gases.





