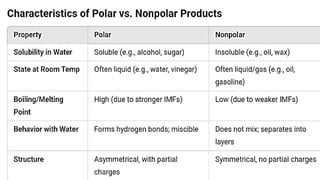
The document provides an overview of intermolecular forces, including dipole-dipole interactions, hydrogen bonding, and London dispersion forces, outlining their definitions and distinctions. It describes how these forces affect physical properties such as boiling points and real-life examples that illustrate their presence and significance. Additionally, practical laboratory activities and observations are included to reinforce understanding of intermolecular forces in action.