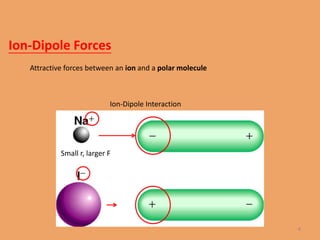
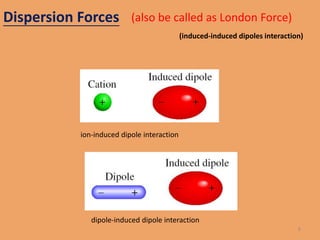
The document discusses the three main types of intermolecular forces: dipole-dipole forces, London dispersion forces, and hydrogen bonding. It describes each type of force, including that dipole-dipole forces exist between polar molecules, London dispersion forces exist between all molecules, and hydrogen bonding specifically refers to the attraction between hydrogen atoms bonded to electronegative atoms like N, O, or F. The document also discusses how these intermolecular forces can explain the physical properties of liquids and some solids.




![7
Hydrogen Bond
A hydrogen bond is the attraction between the lone pair of an
electronegative atom and a hydrogen atom that is bonded to either
nitrogen, oxygen, or fluorine.[3] The hydrogen bond is often described
as a strong electrostatic dipole-dipole interaction. However, it also has
some features of covalent bonding: it is directional, stronger than a
van der Waals interaction, produces interatomic distances shorter
than the sum of van der Waals radius, and usually involves a limited
number of interaction partners, which can be interpreted as a kind of
valence.](https://image.slidesharecdn.com/intermolecularforces-150902162839-lva1-app6891/85/Intermolecular-forces-7-320.jpg)



