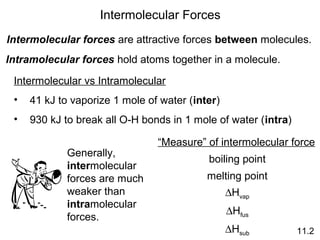
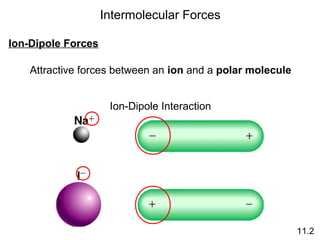
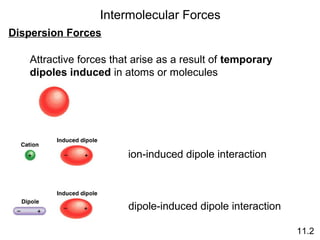
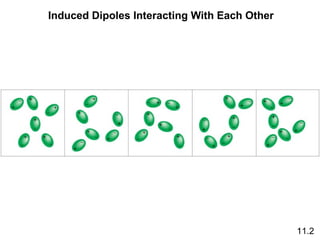
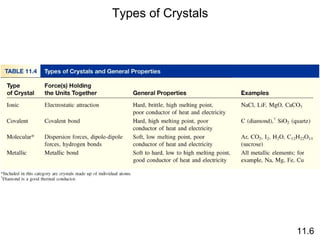
The document discusses intermolecular forces, the different types of intermolecular forces (dipole-dipole, ion-dipole, dispersion), and hydrogen bonding. It also covers properties of liquids related to intermolecular forces like surface tension, viscosity, and water's unusual properties. Finally, it discusses phases of matter, phase transitions, vapor pressure, boiling/melting points, and heat effects of phase changes.