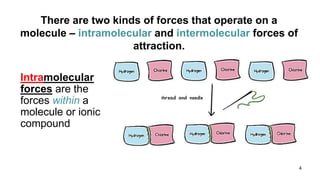
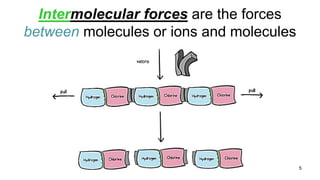
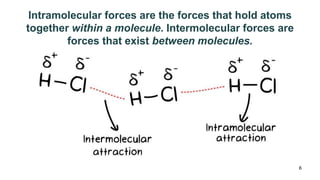
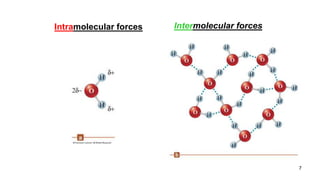
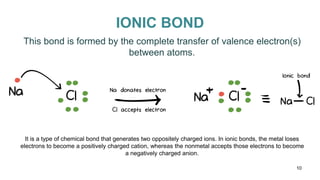
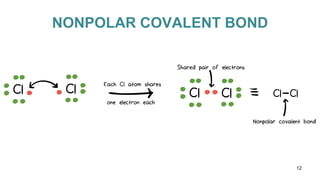
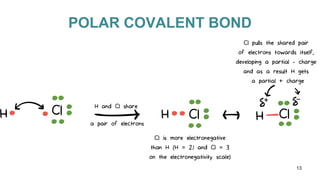
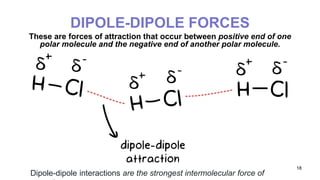
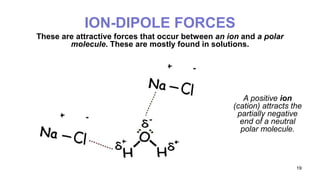
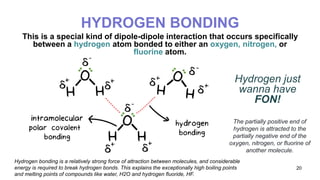
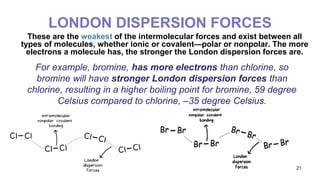
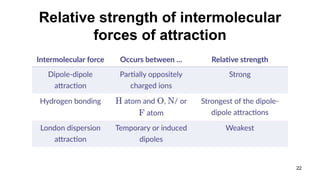
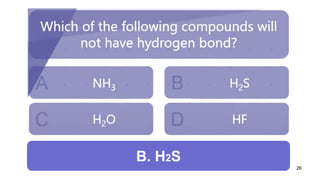
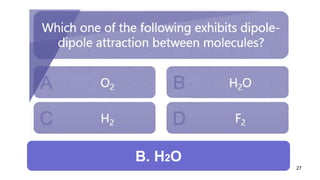
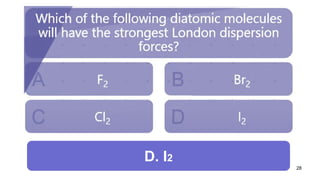
This document discusses intramolecular and intermolecular forces. It defines intramolecular forces as those within a molecule that hold atoms together, such as ionic, covalent, and metallic bonds. Intermolecular forces are weaker forces between molecules, including dipole-dipole, ion-dipole, hydrogen bonding, and London dispersion forces. The document explains each type of intramolecular and intermolecular force in more detail and notes that intramolecular bonds are stronger than intermolecular forces.