Embed presentation
Downloaded 559 times



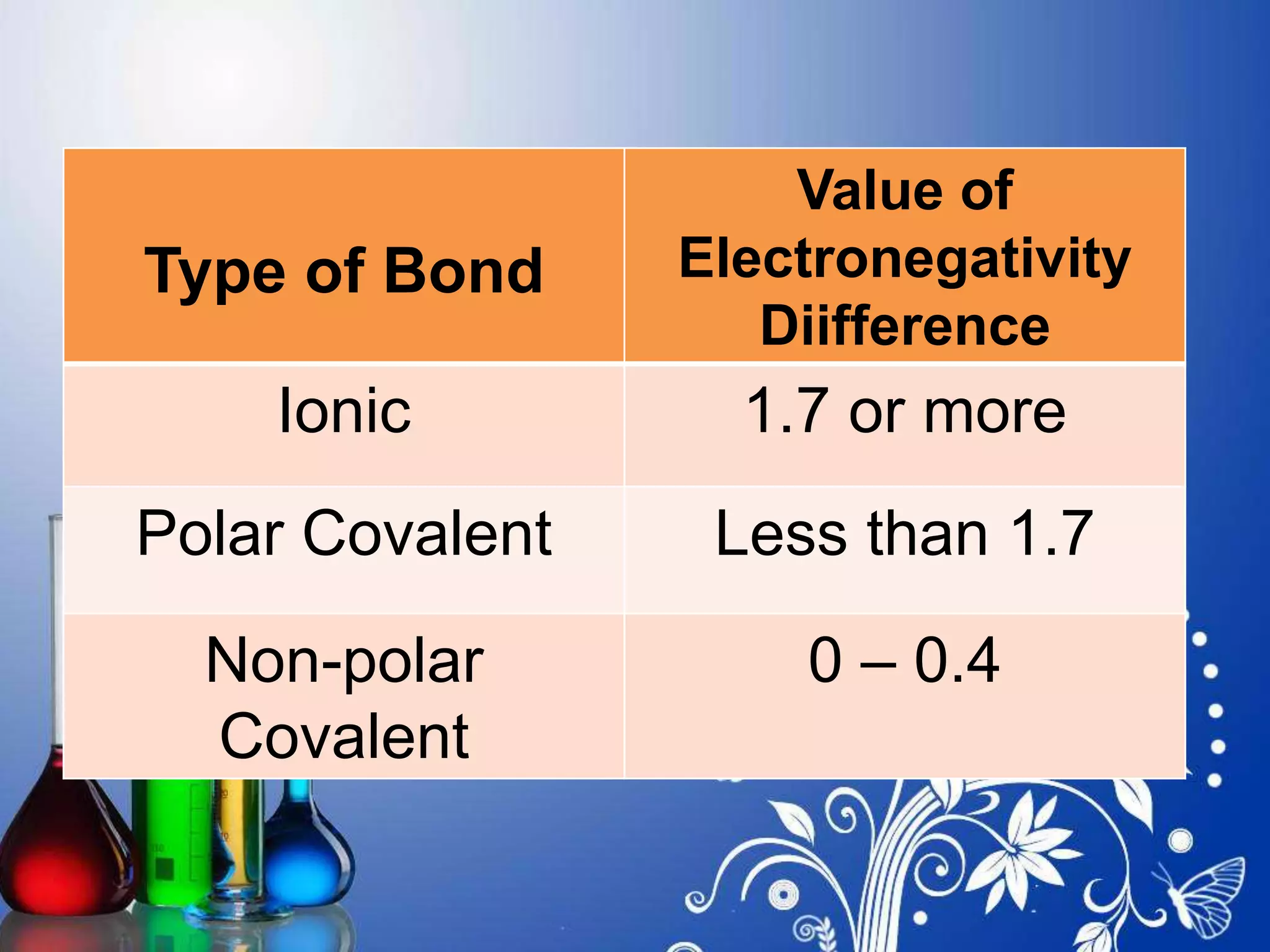


The document explains the differences between polar and non-polar covalent bonds, highlighting that polar bonds involve unequal sharing of electrons while non-polar bonds involve equal sharing. It lists examples of each type of bond and describes how the type can be determined through electronegativity differences between atoms. Specific electronegativity value thresholds are provided to classify bonds as ionic, polar covalent, or non-polar covalent.




