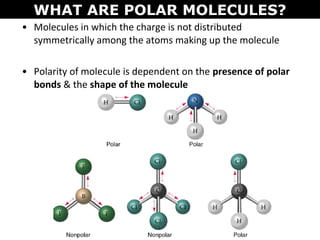
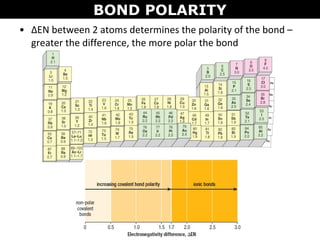
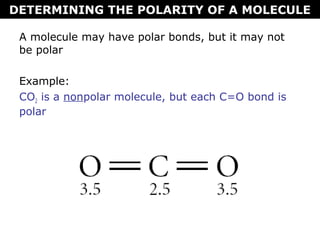
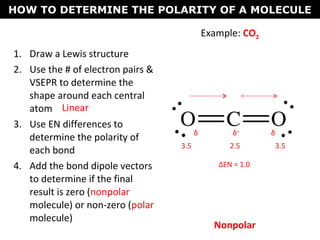
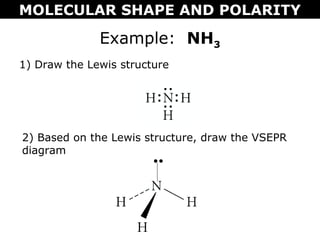
This document discusses molecular shape and polarity. It explains that a molecule's polarity depends on the presence of polar bonds and its overall shape. Polar bonds form when there is an unequal sharing of electrons between atoms. To determine a molecule's polarity, one must draw its Lewis structure, use VSEPR theory to predict its shape, determine if bonds are polar, then add bond dipoles. If dipoles cancel out, the molecule is nonpolar. If not, it is polar. Symmetry is also important - symmetrical molecules with identical bond polarities are nonpolar. Examples include the polar NH3 and nonpolar CO2 and symmetrical NF3.