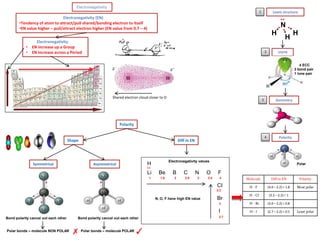
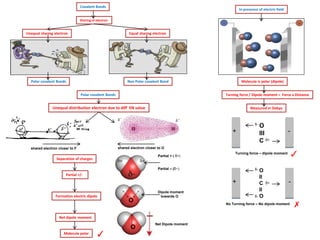
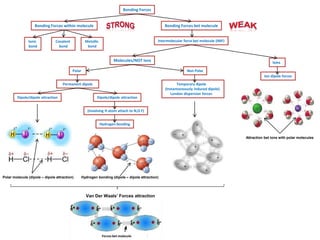
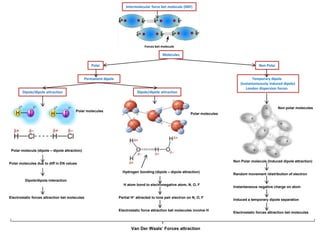
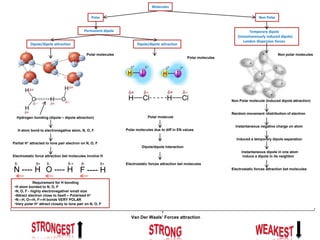
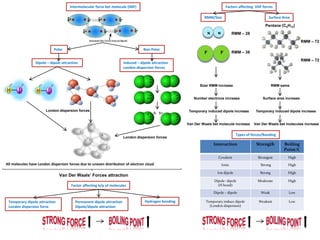
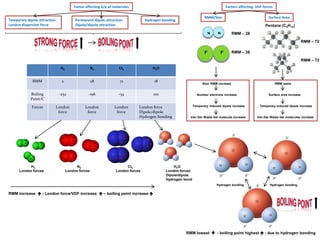
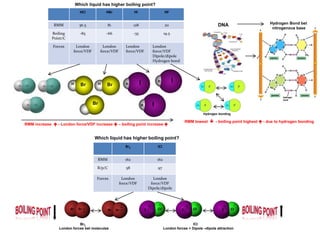
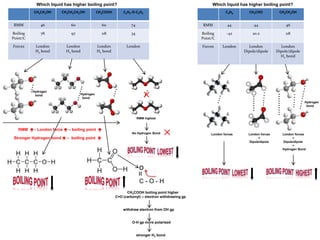
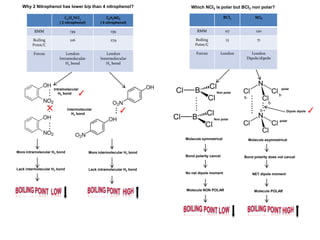
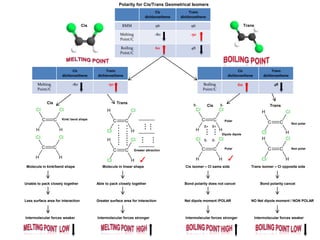
- Ionic bonds form when a metal transfers an electron to a nonmetal, forming positive and negative ions.
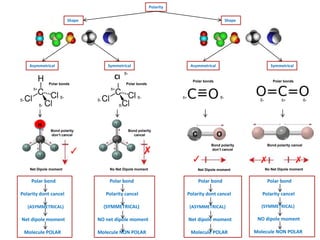
- Covalent bonds form when atoms share electrons, either equally in nonpolar covalent bonds or unequally in polar covalent bonds.
- The electronegativity difference between bonded atoms determines bond polarity.