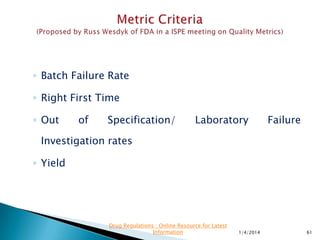
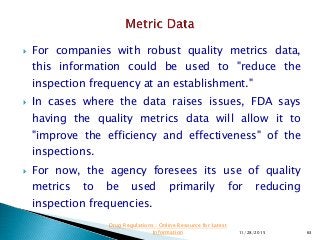
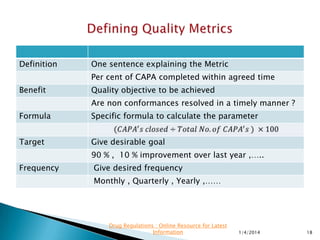
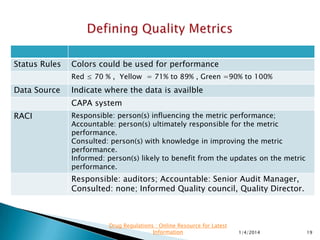
This presentation by 'Drug Regulations' focuses on providing pharmaceutical professionals with updated guidance on quality metrics, emphasizing the importance of selecting appropriate performance metrics that align with overall strategic objectives. It covers the nature of quality metrics, the balance between leading and lagging indicators, and the significance of accountability, visibility, and problem-solving in quality management. Additionally, the presentation discusses the evaluation of supplier performance and compliance, advocating for systematic approaches to establish a robust quality measurement system.
















































![ FDA plans to use its authority to collect records
"in advance of or in lieu of" an inspection,
under section 704(a)(4)(A) of the FD&C Act to
gather various quality metrics data records.
The agency says it will use these records to
"further develop [its] risk-based inspection
scheduling."
11/28/2015 57
Drug Regulations : Online Resource for Latest
Information](https://image.slidesharecdn.com/qualitymerics-140104053554-phpapp01/85/Quality-Metrics-57-320.jpg)