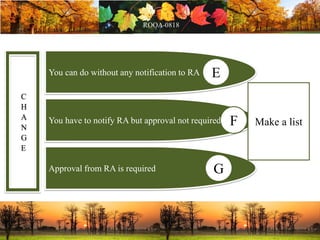
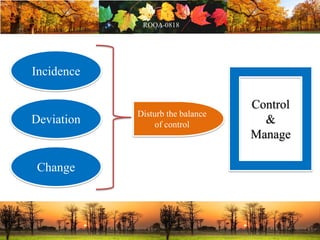
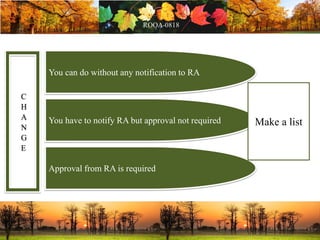
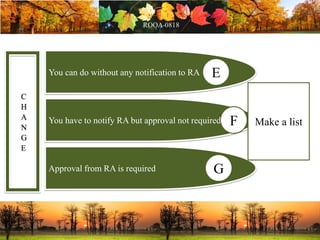
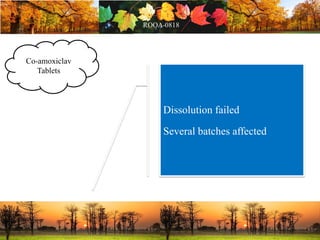
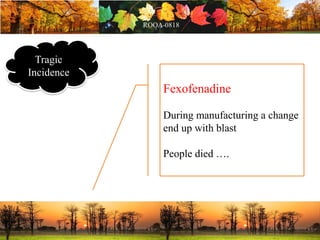
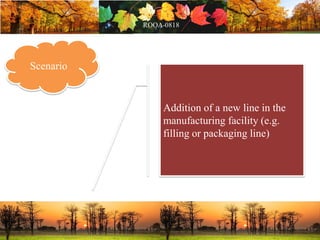
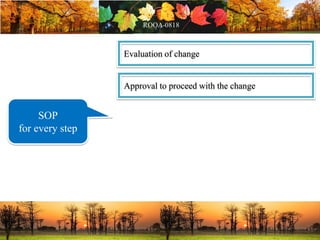
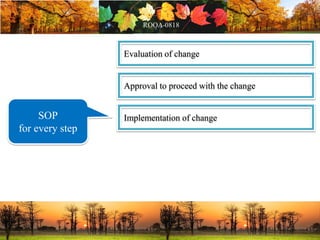
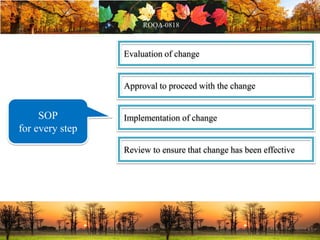
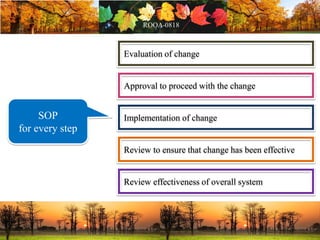
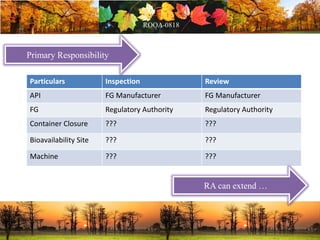
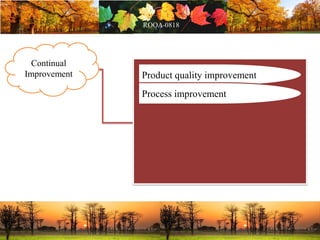
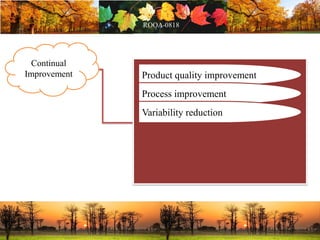
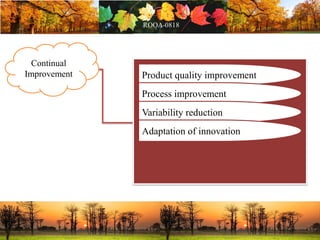
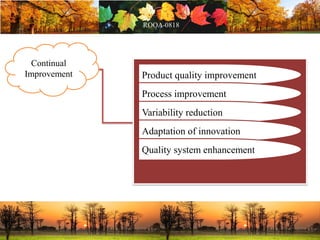
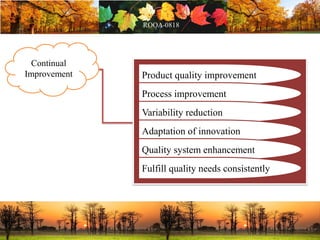
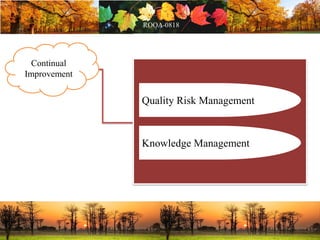
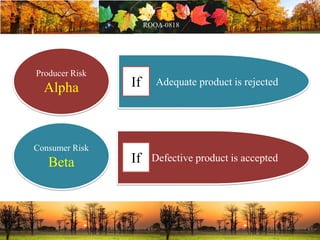
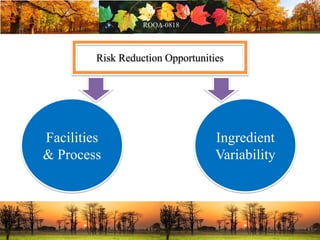
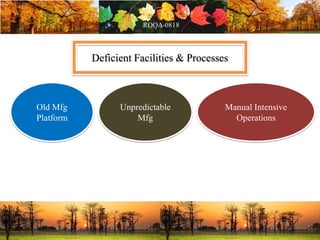
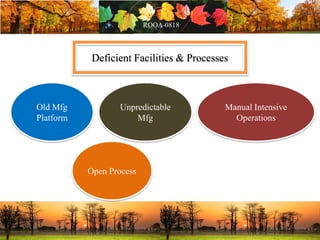
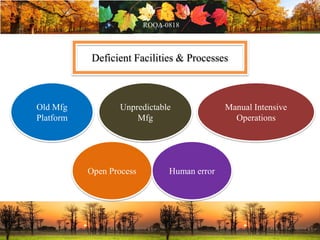
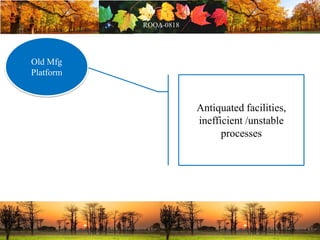
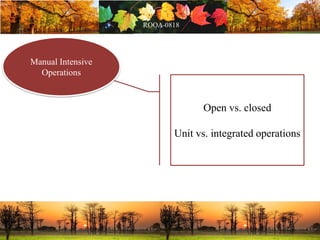
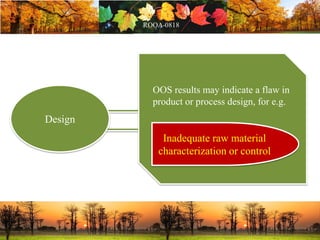
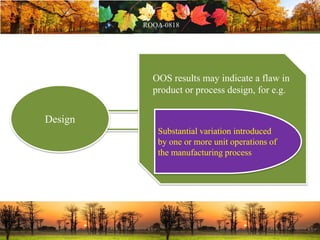
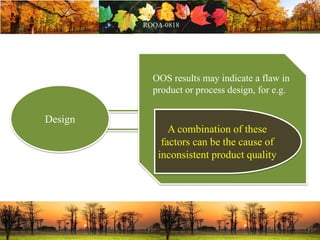
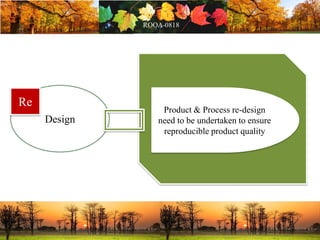
This document discusses change management in pharmaceutical manufacturing, emphasizing regulatory compliance and real-world impacts of changes in processes, equipment, and materials on product quality. It highlights the need for effective quality management systems, proper risk assessment, and ongoing evaluation to ensure safety and regulatory adherence. Various case studies illustrate the severe consequences of inadequate change control, underscoring the importance of maintaining a state of control throughout the product lifecycle.