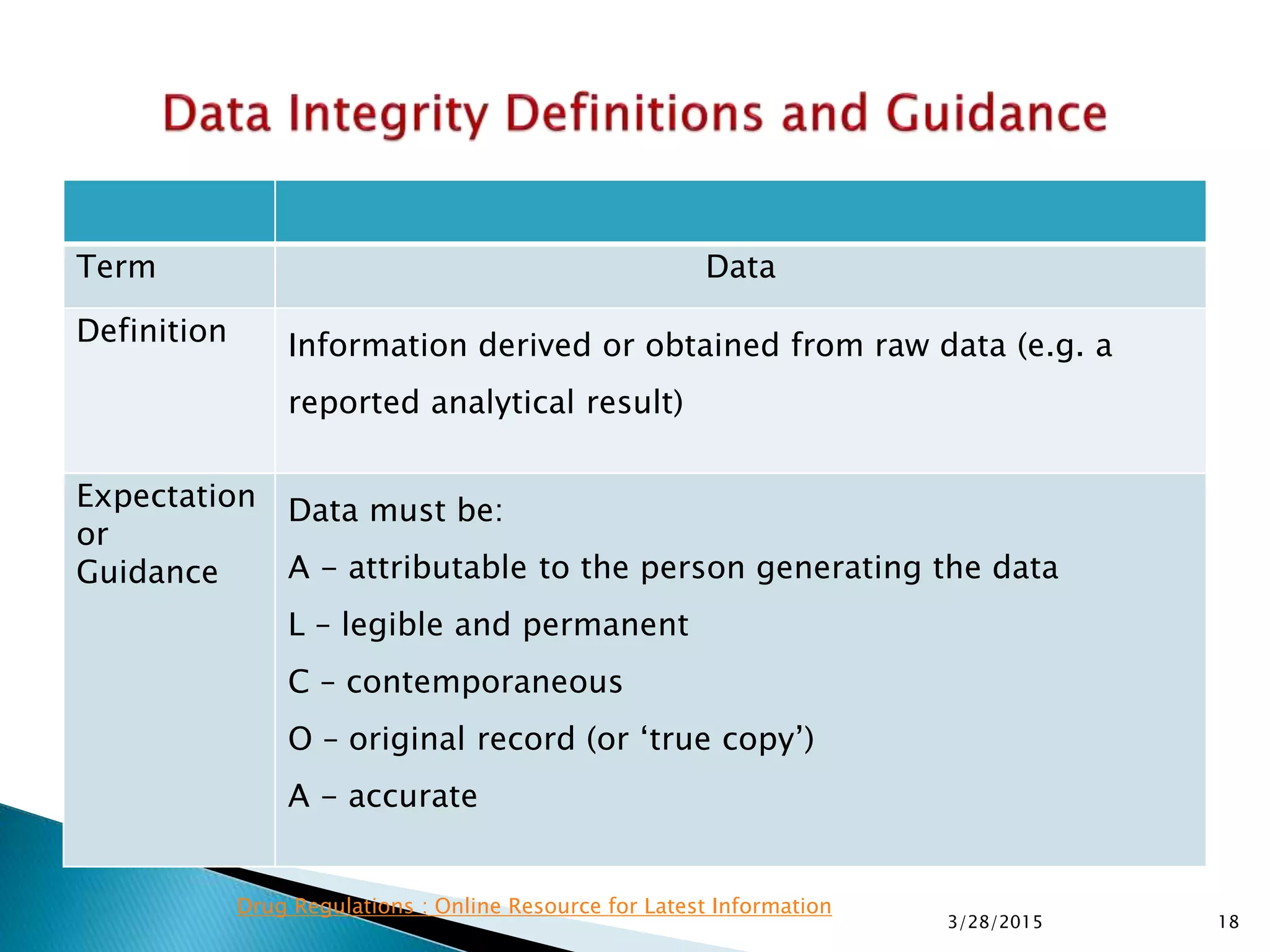
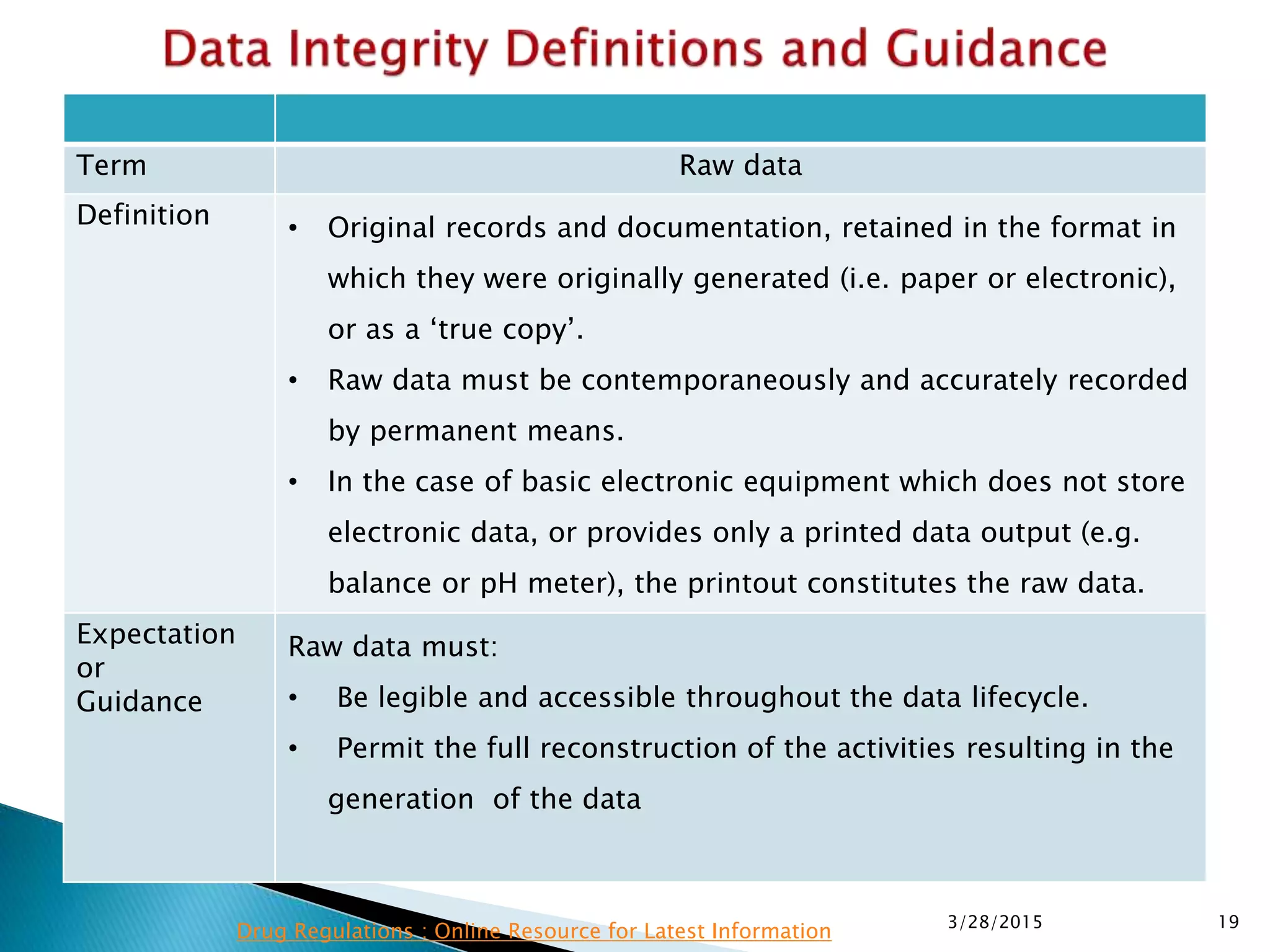
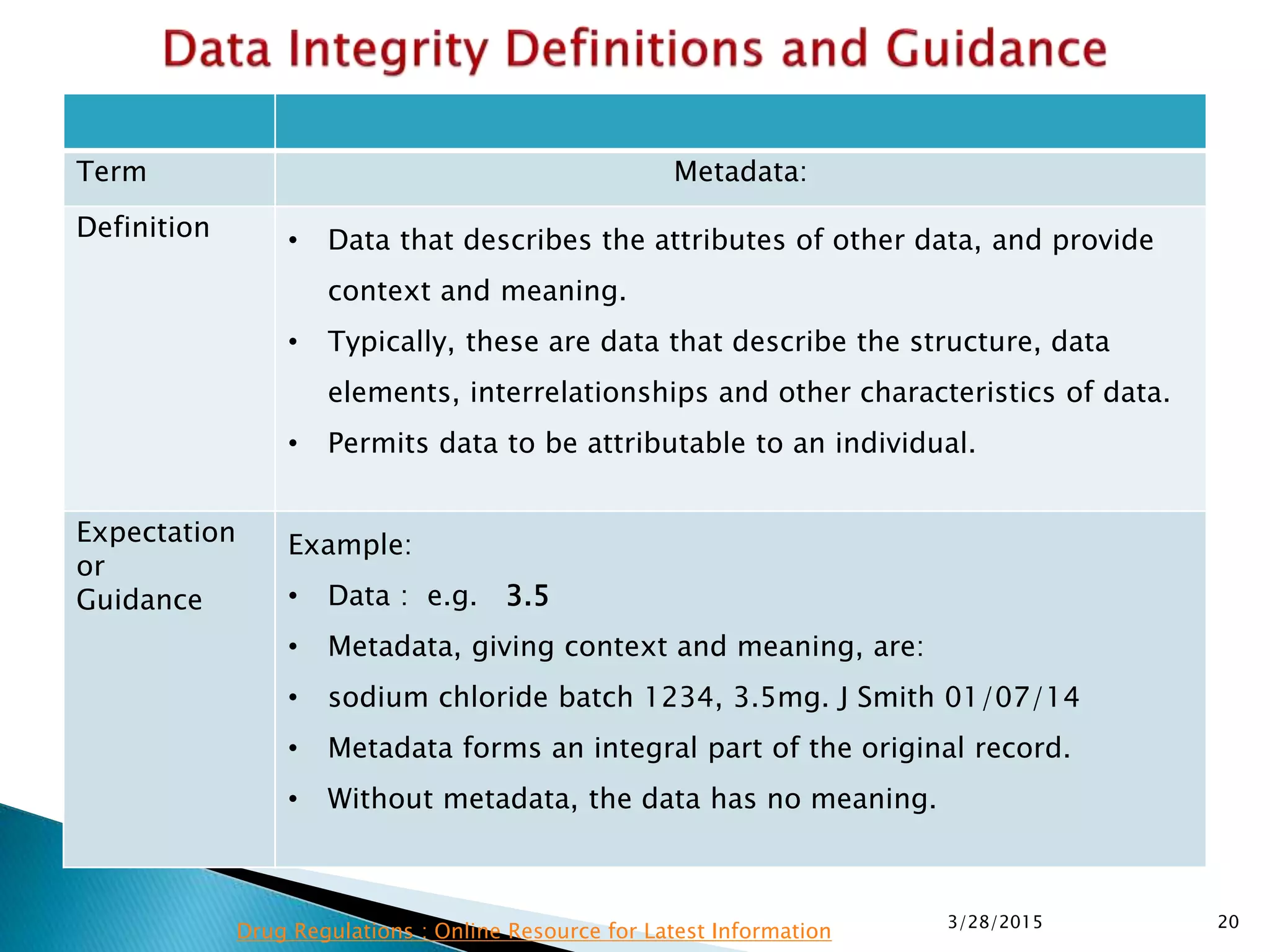
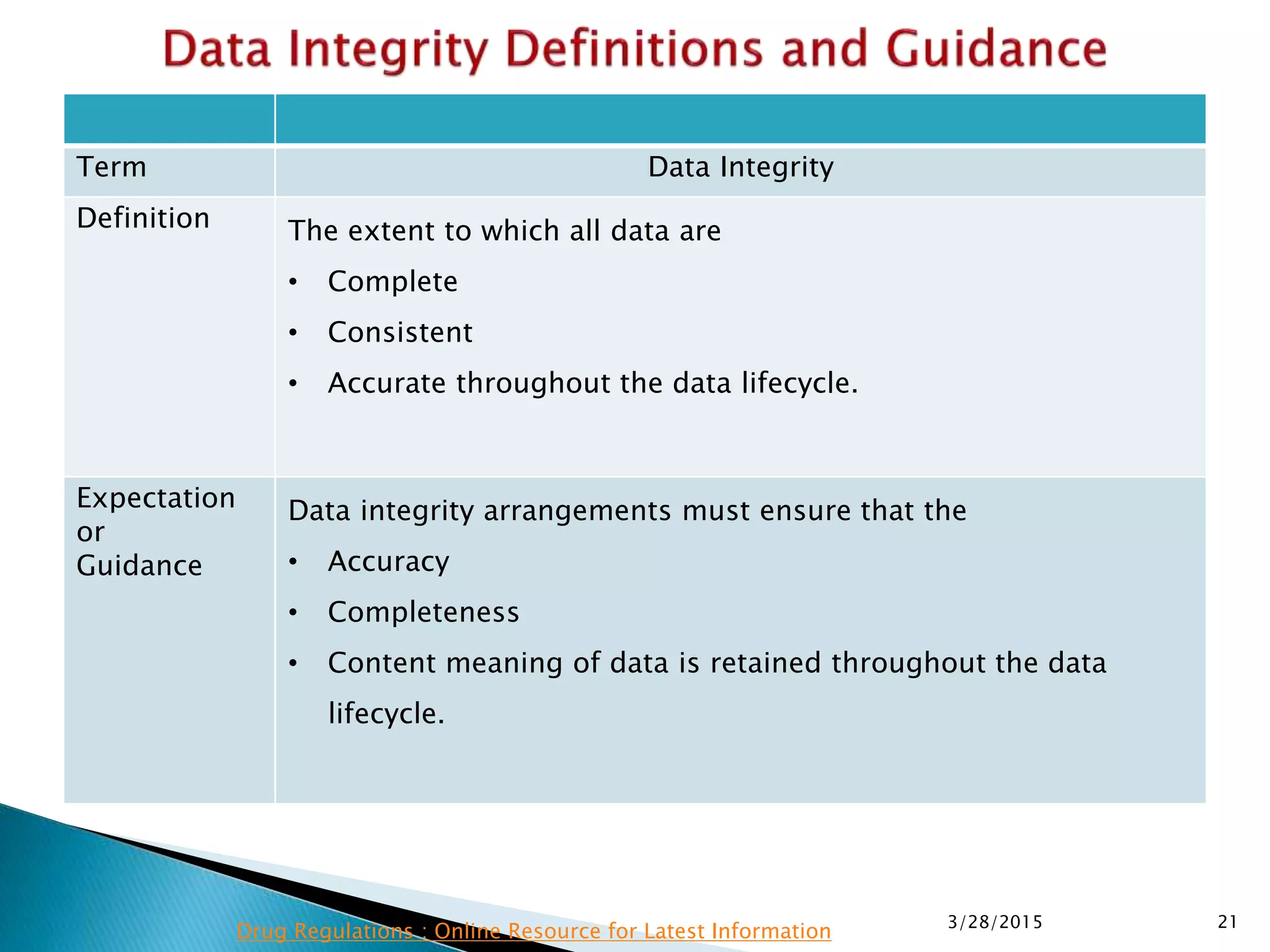
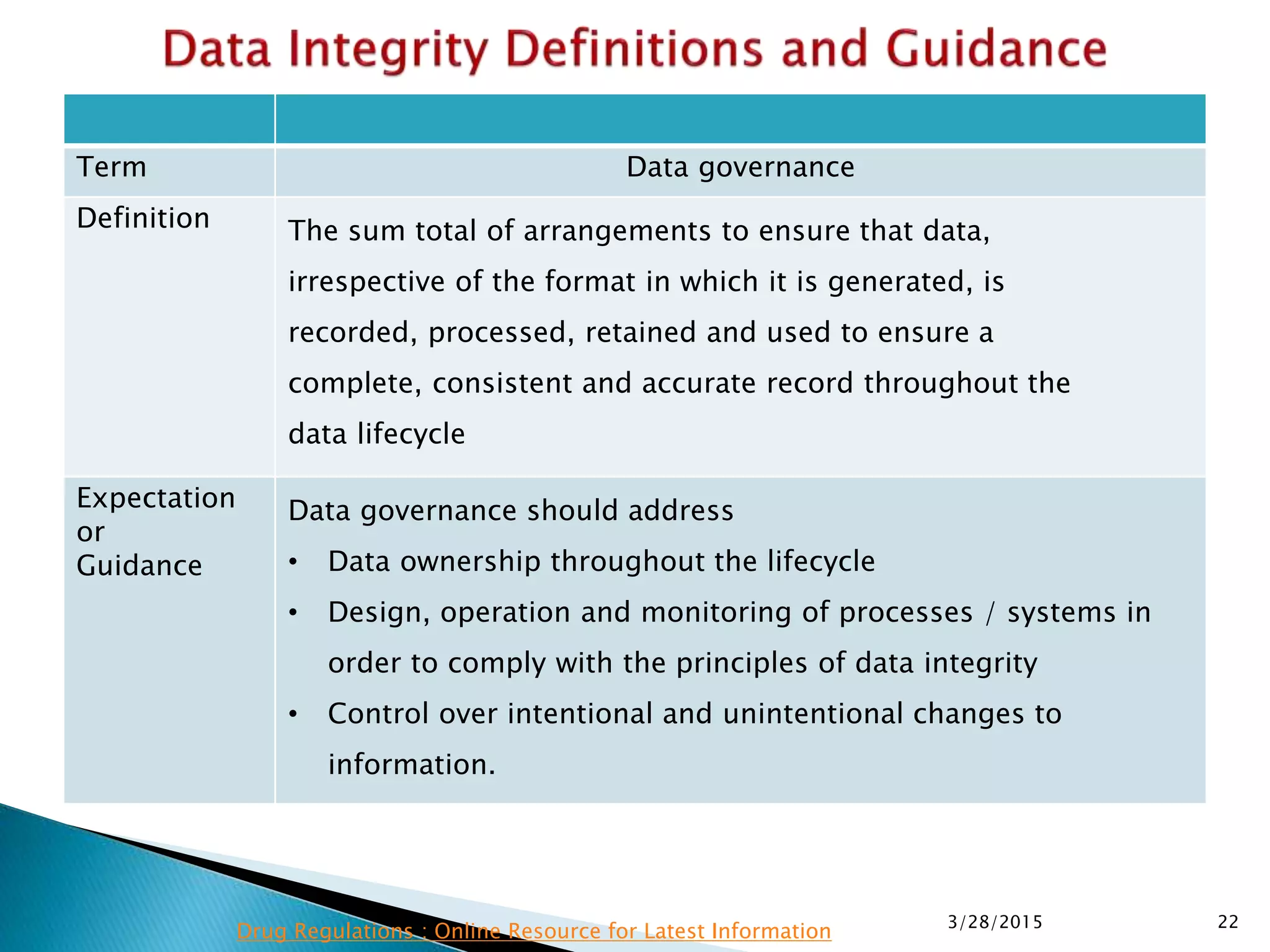
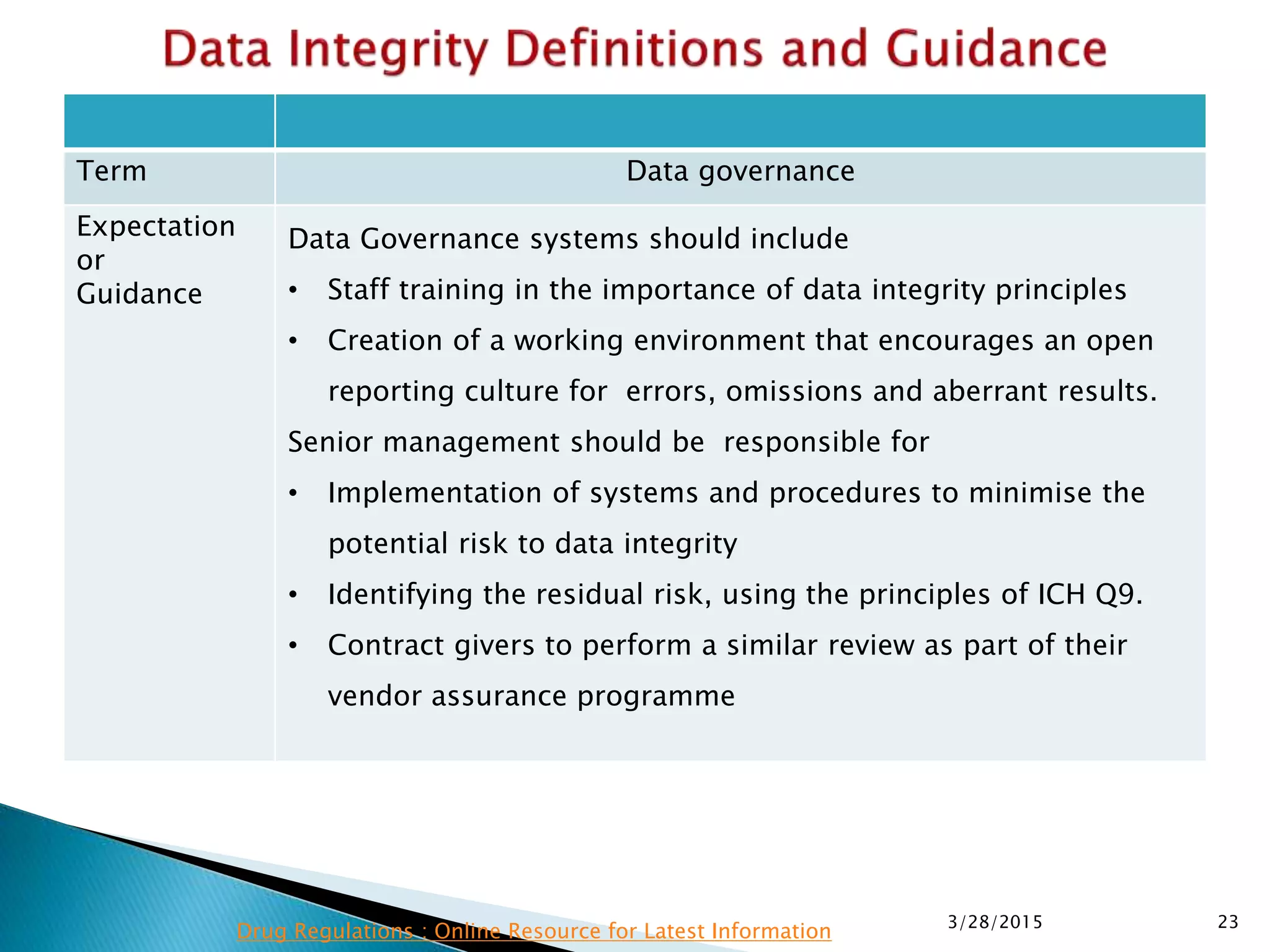
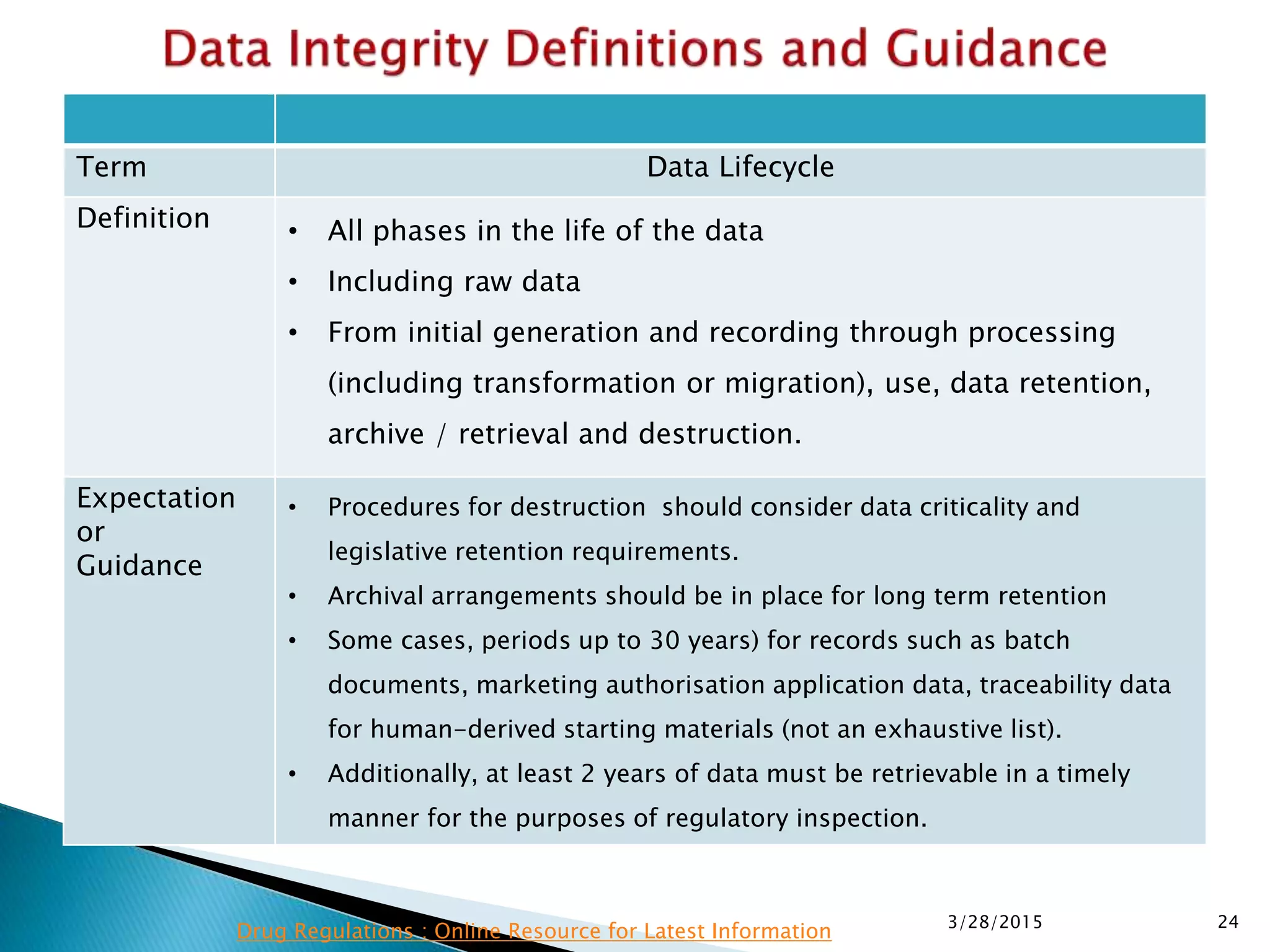
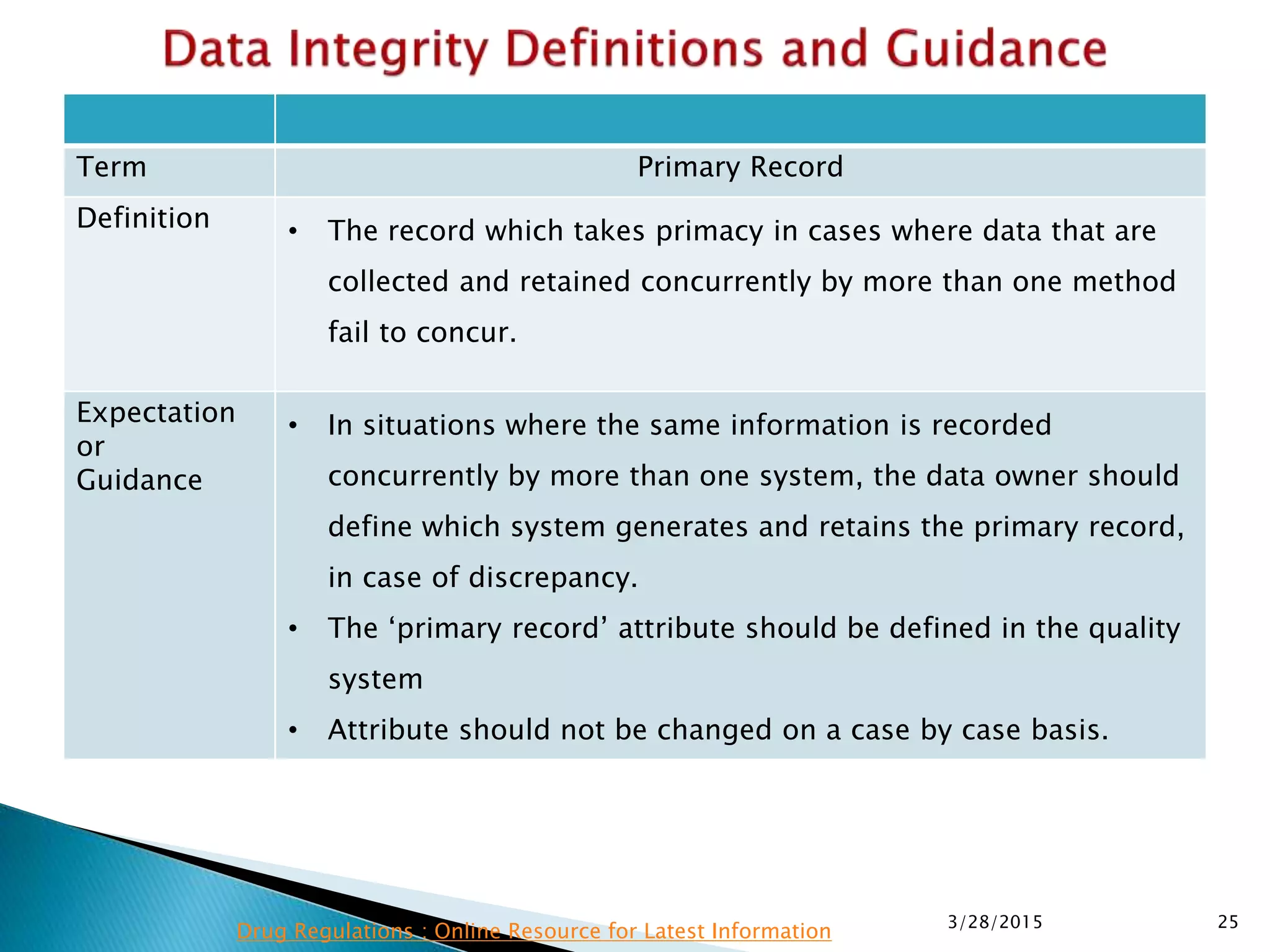
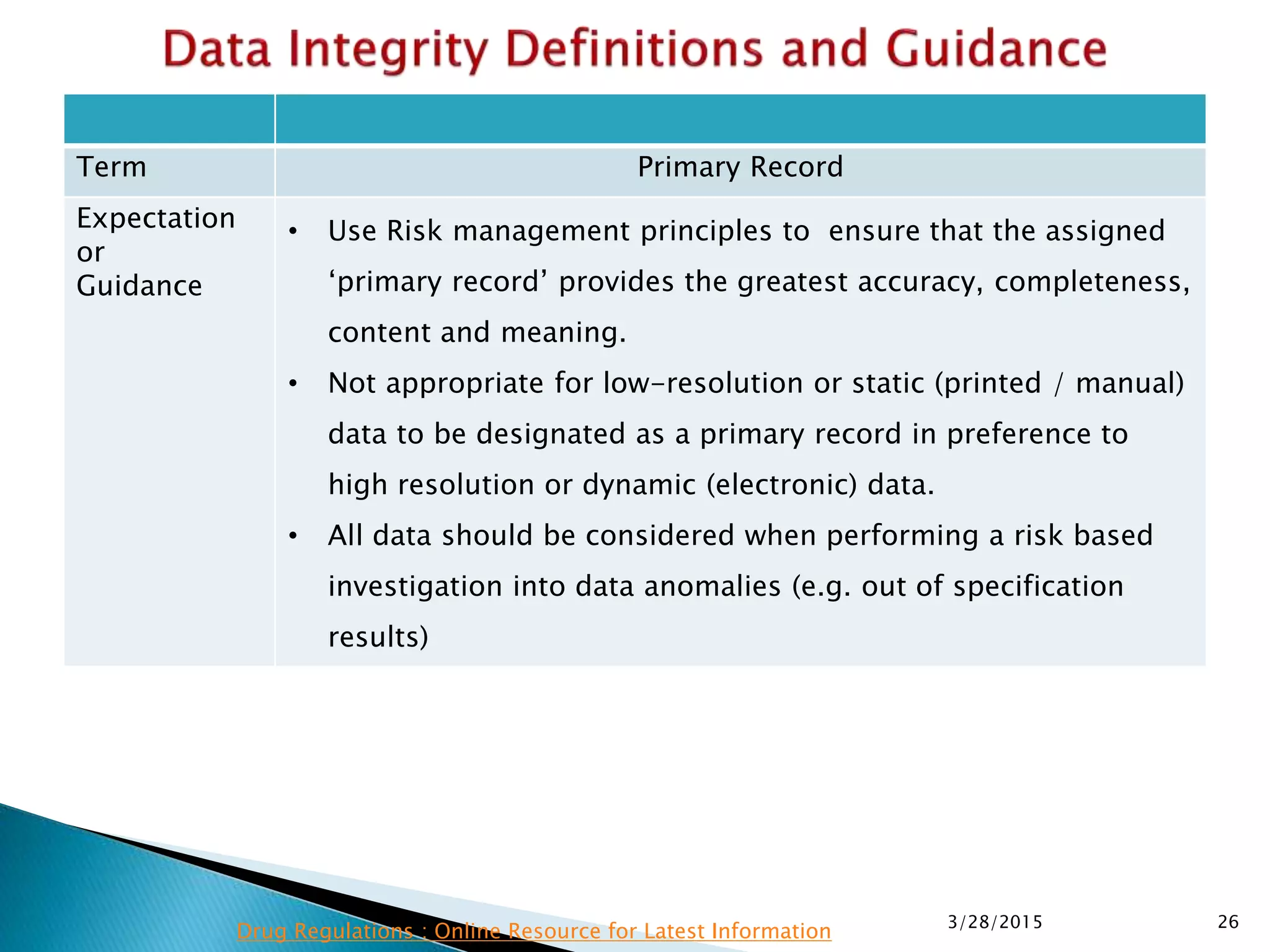
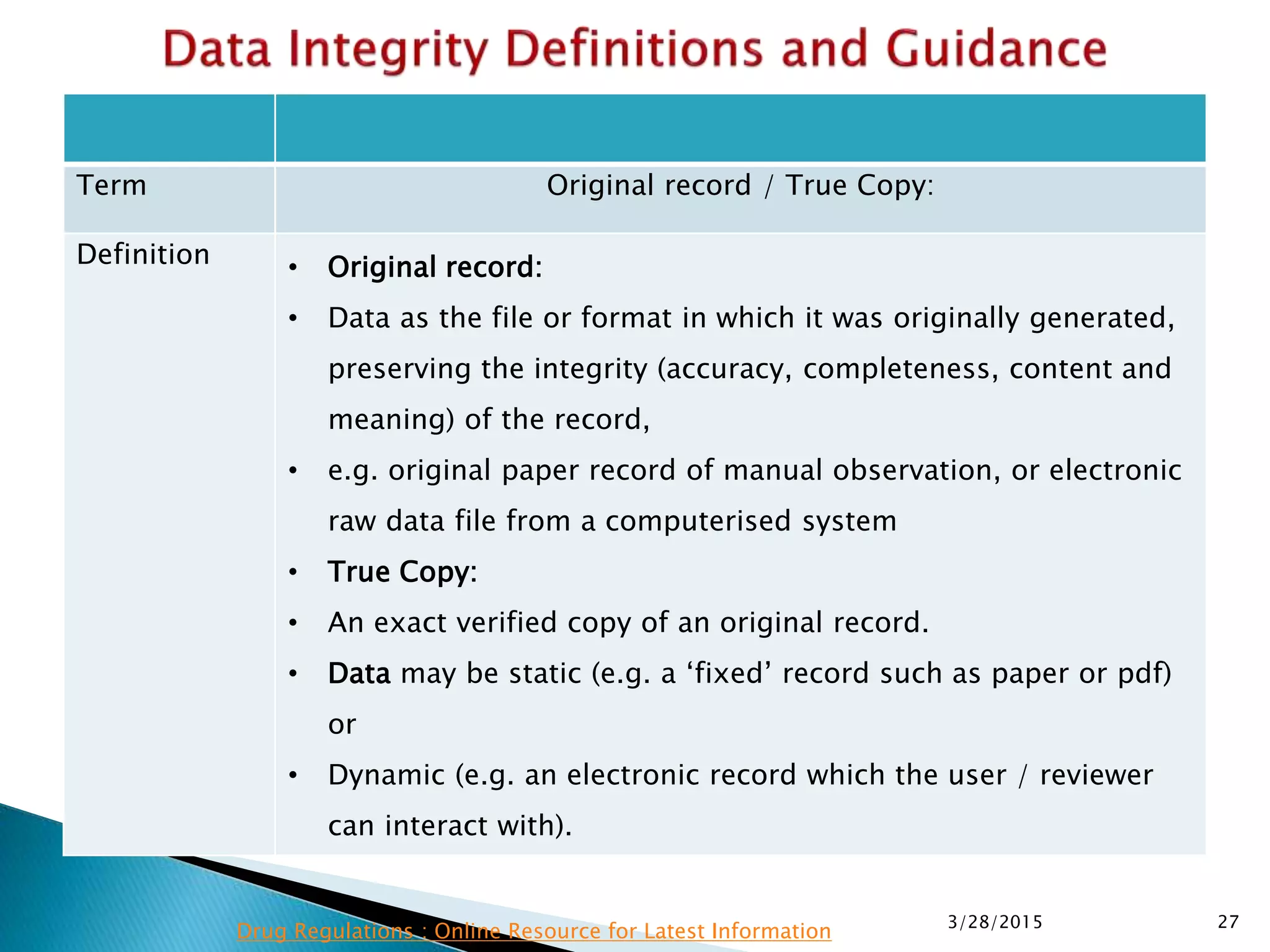
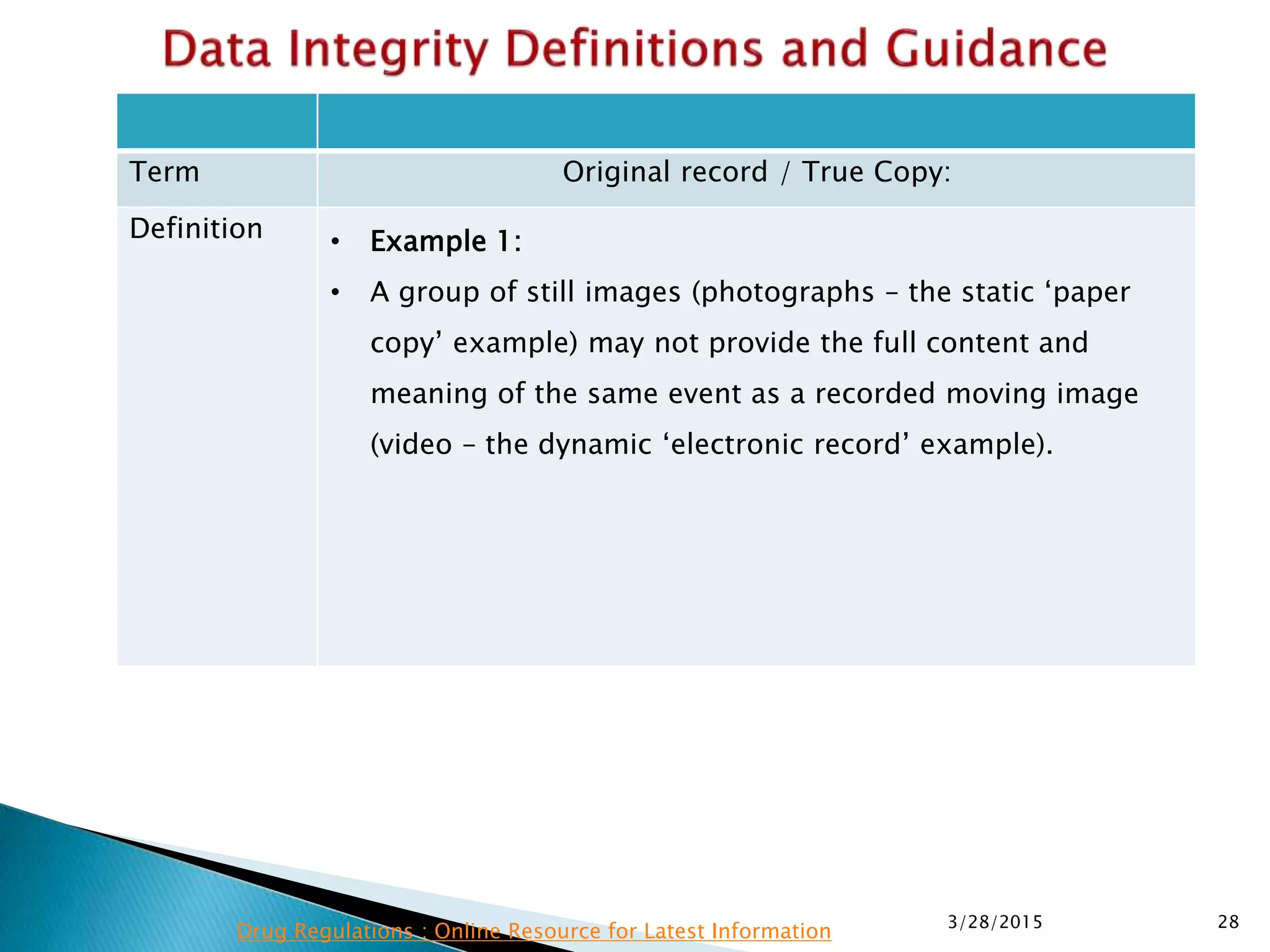
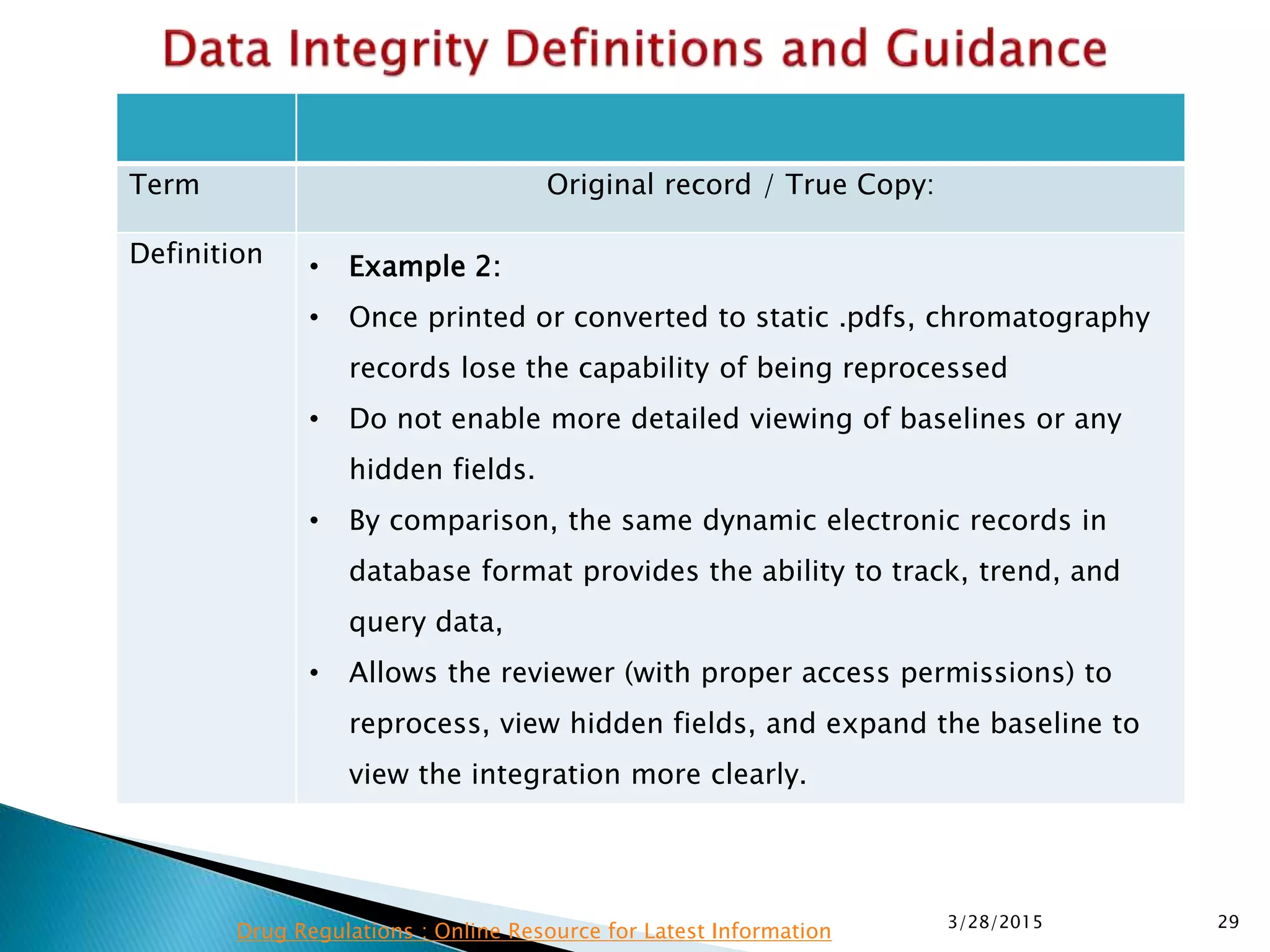
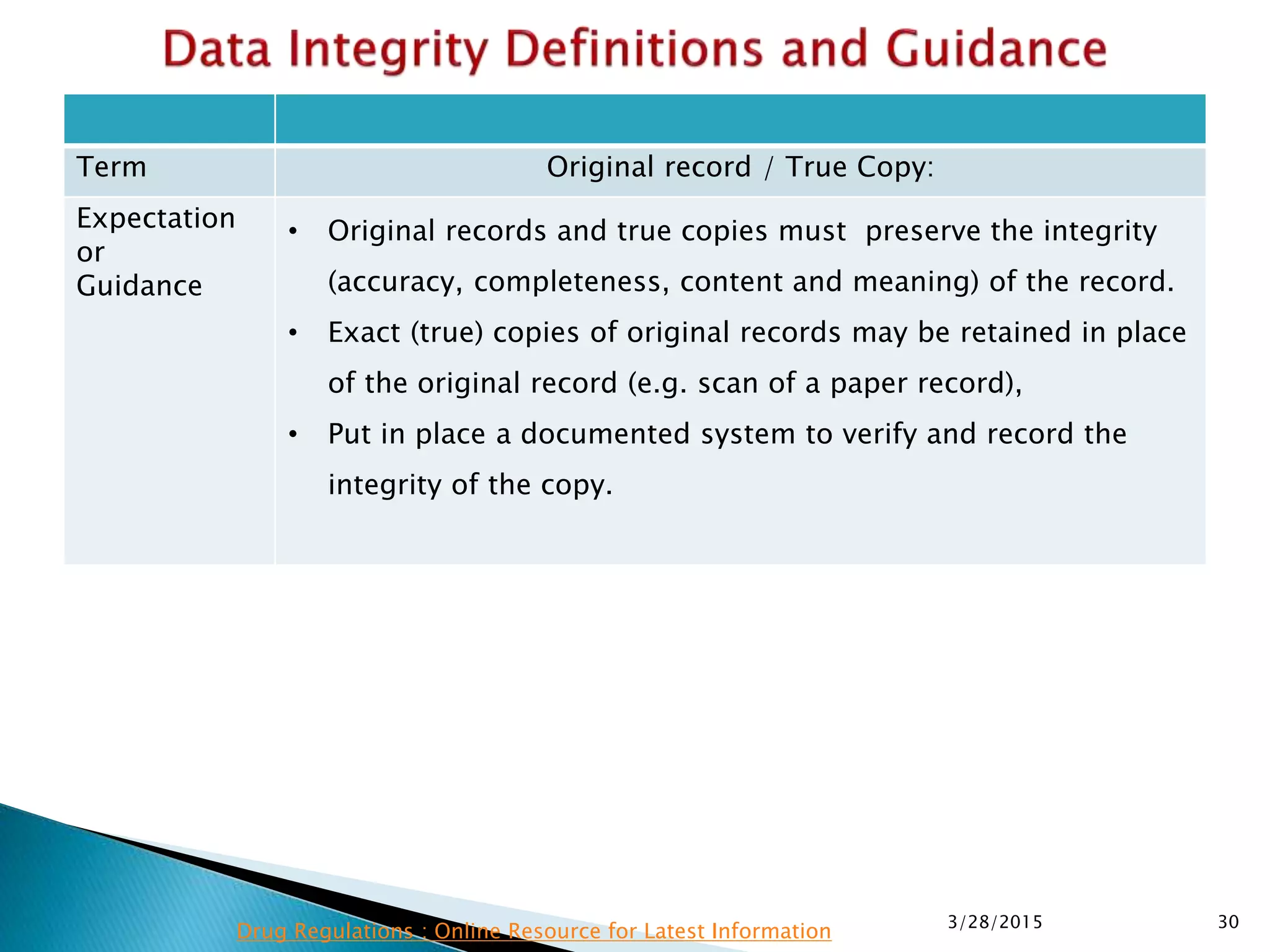
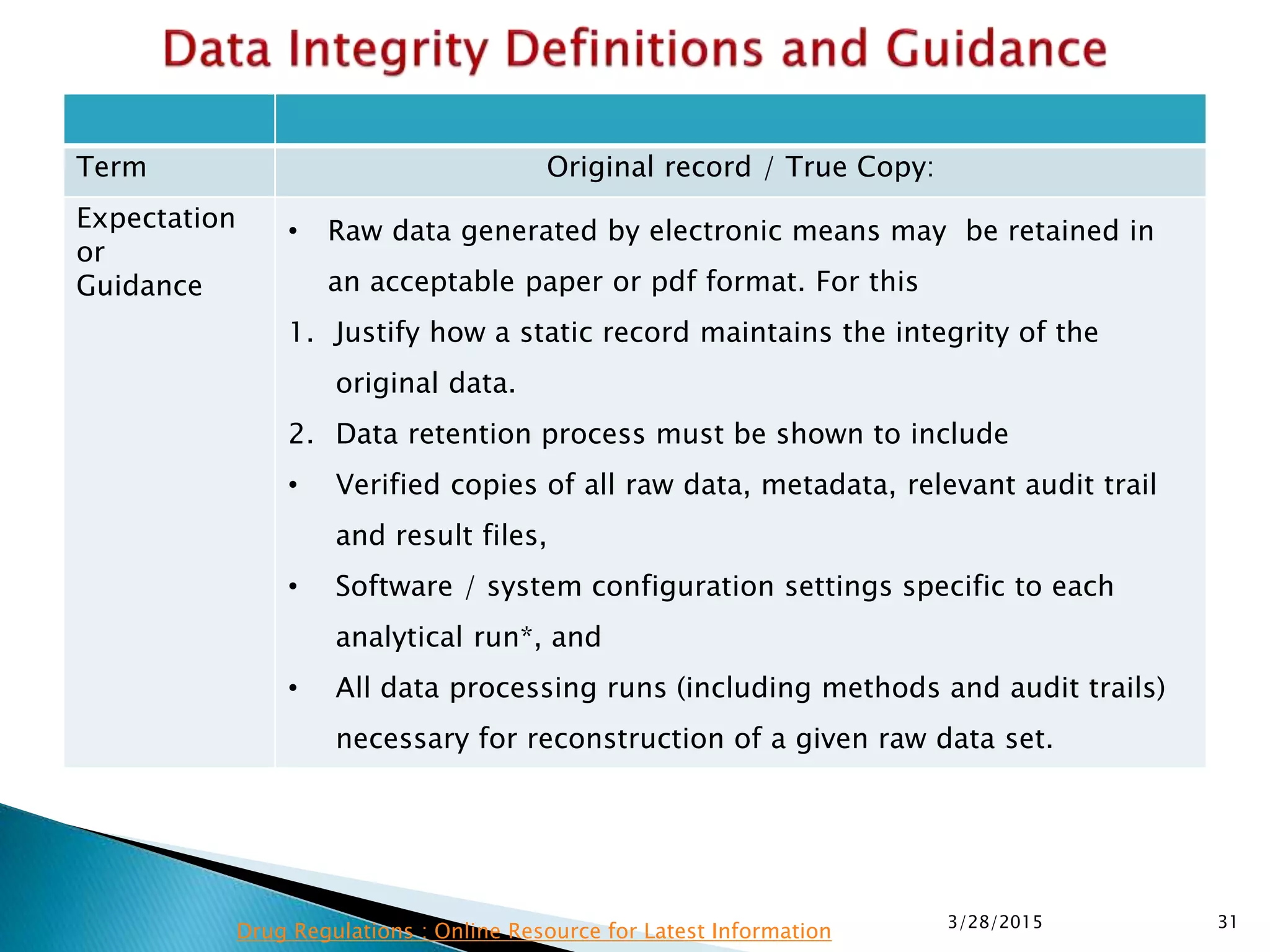
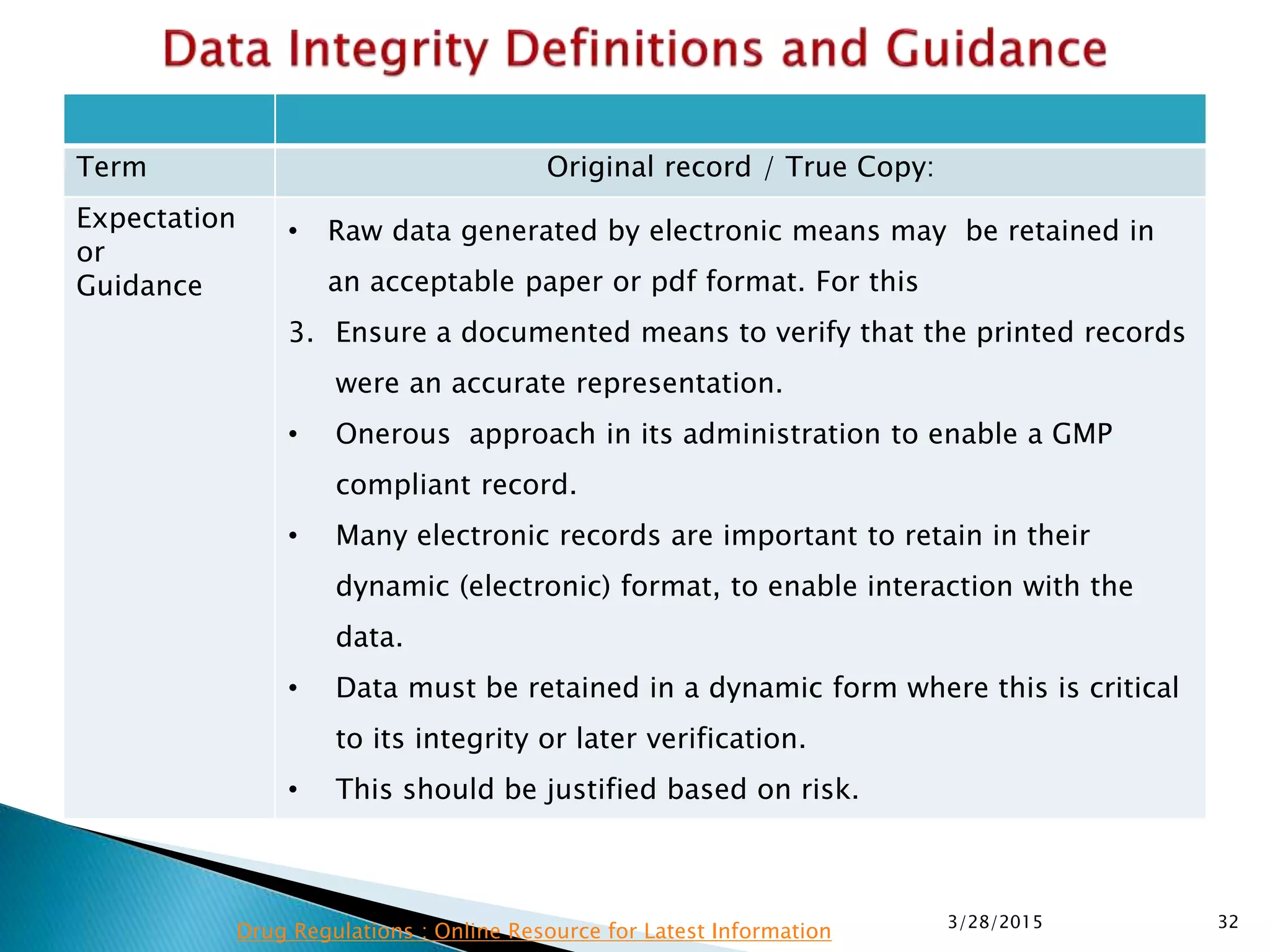
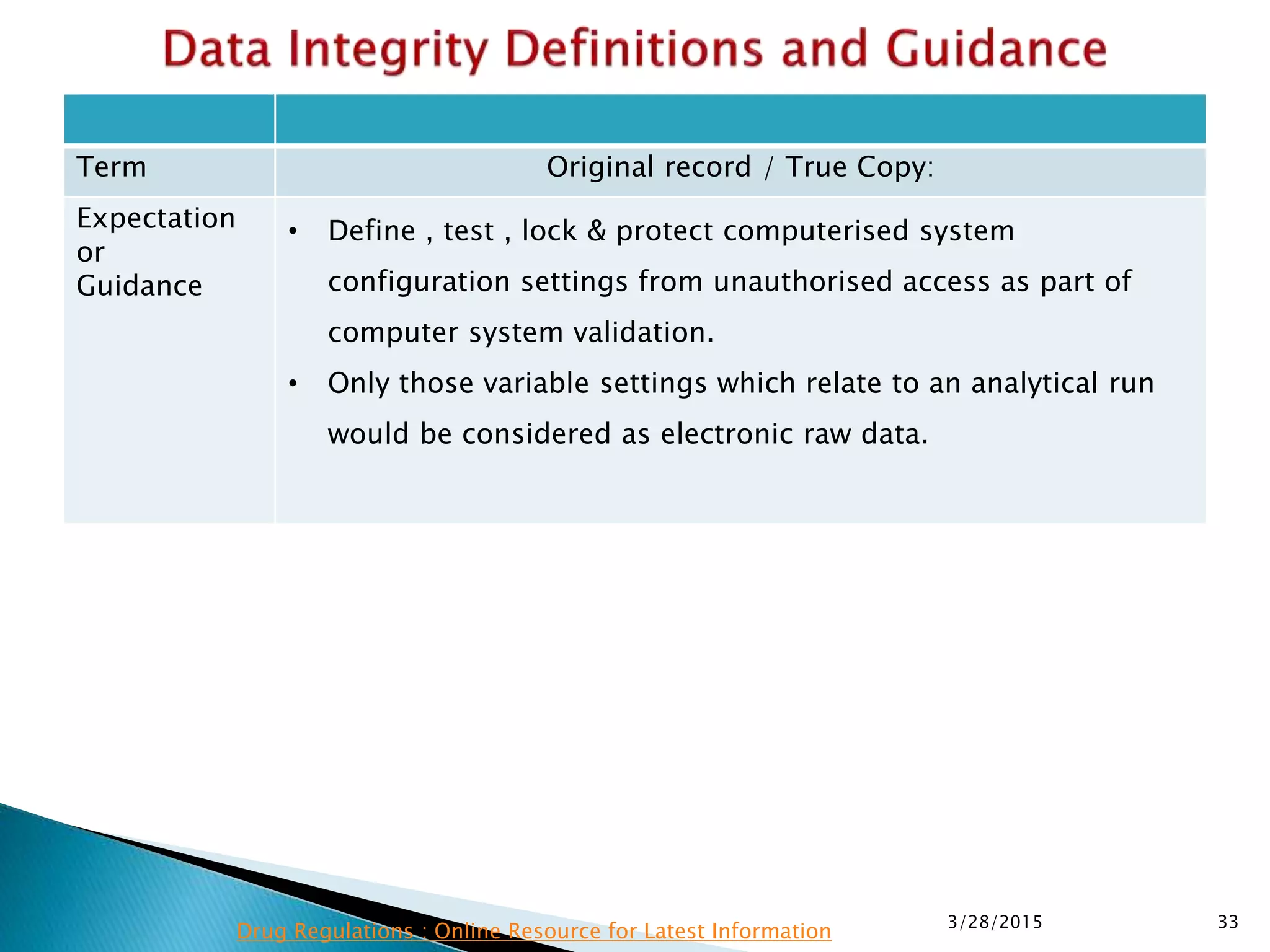
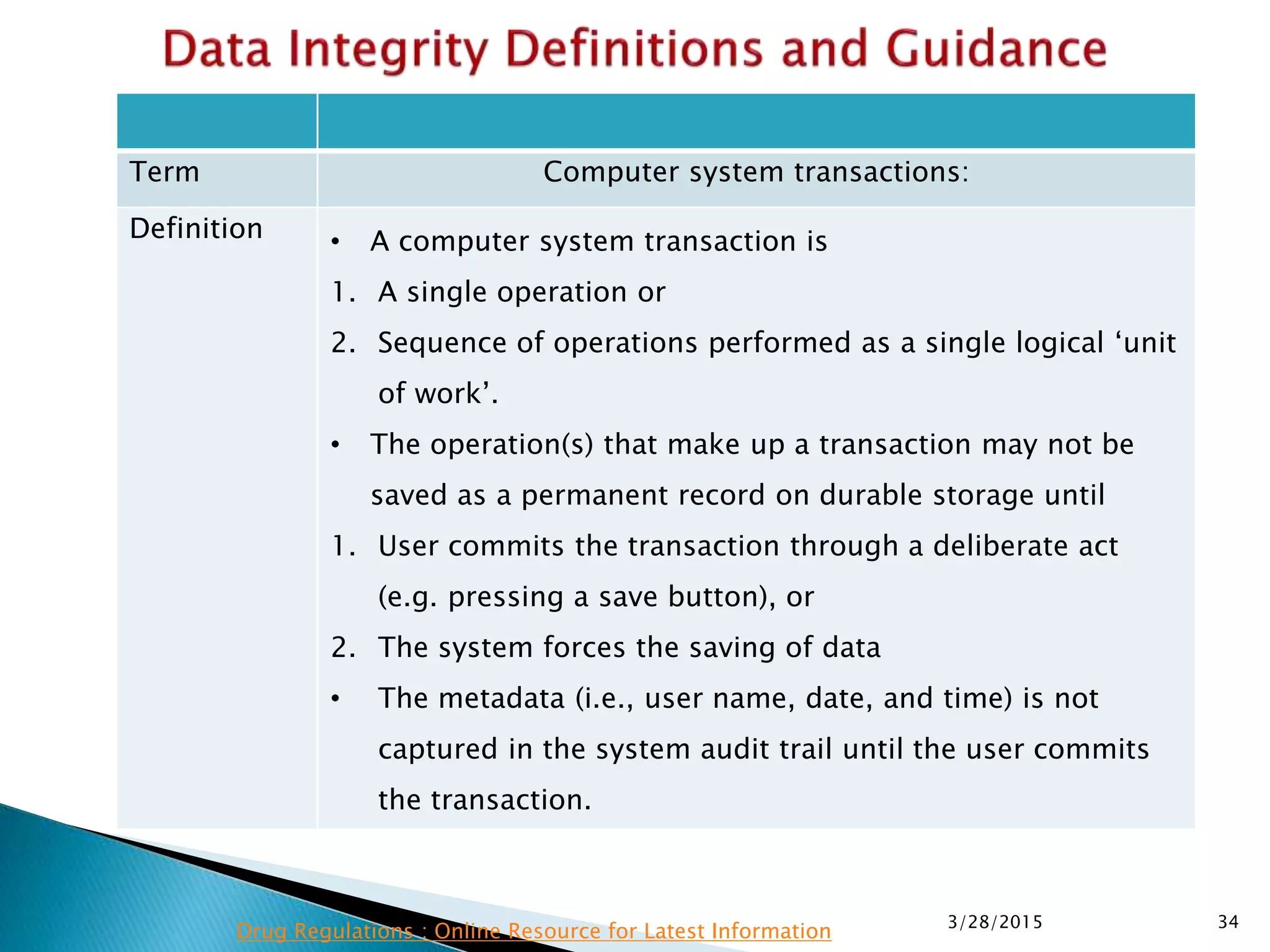
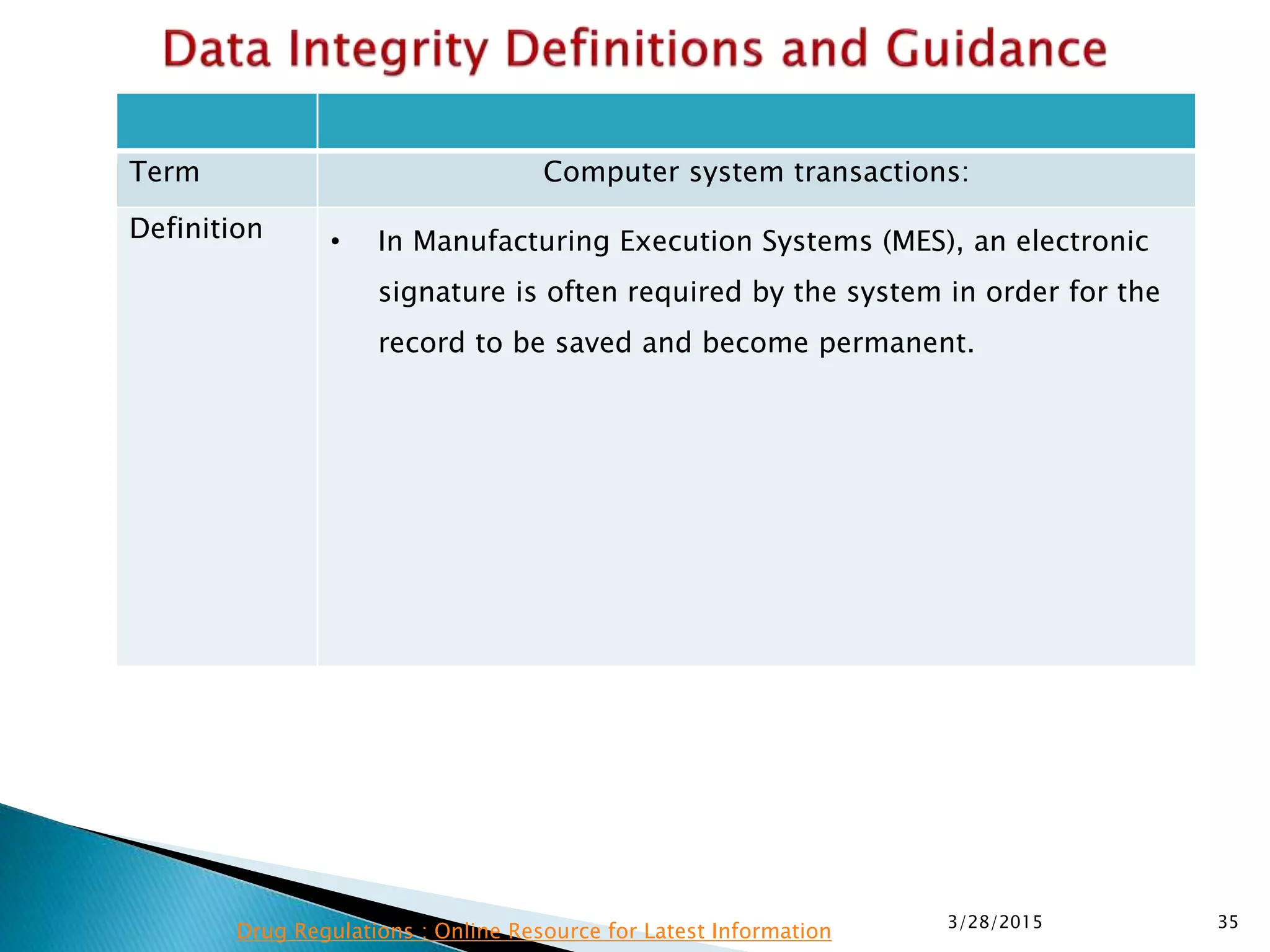
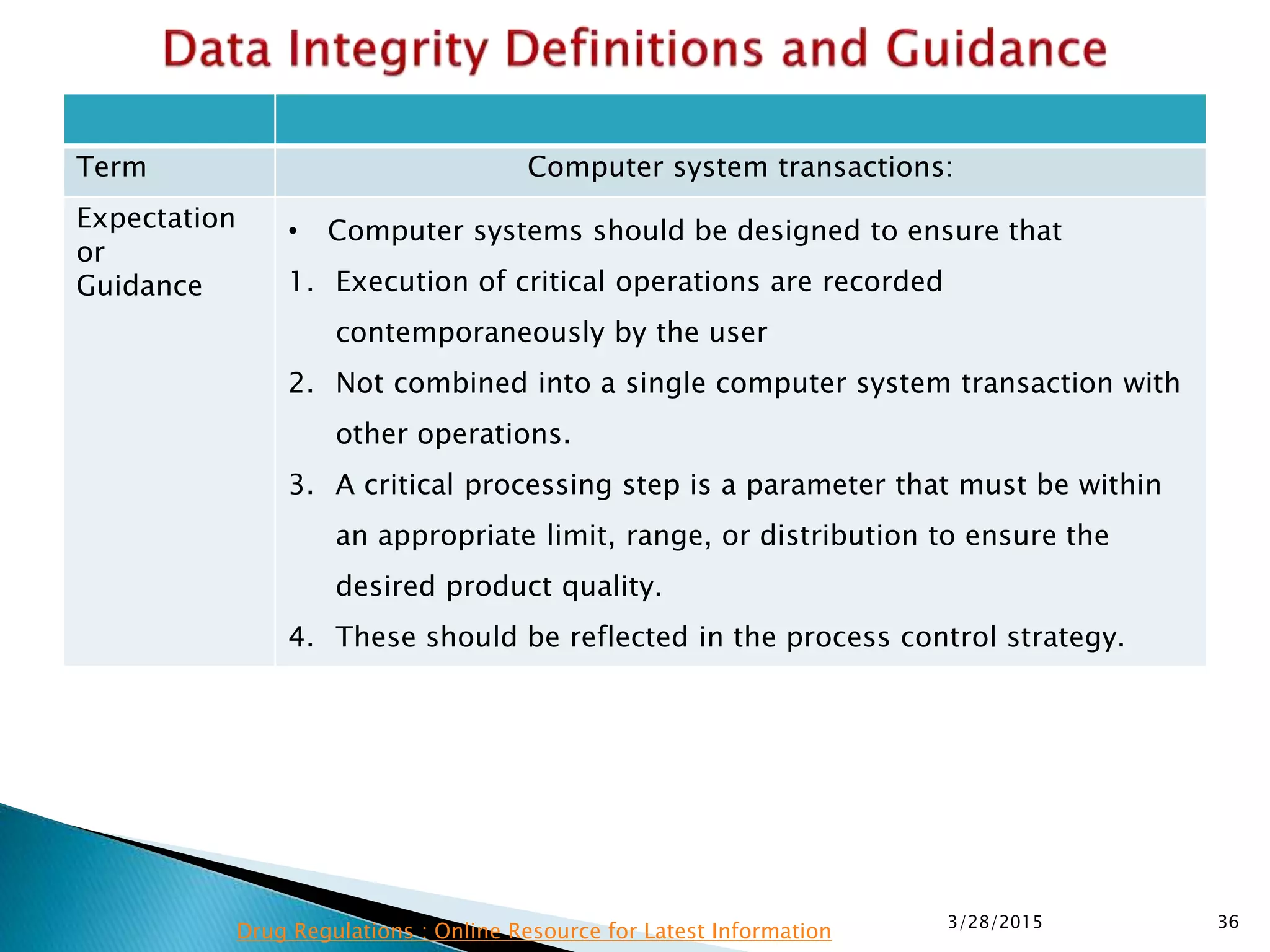
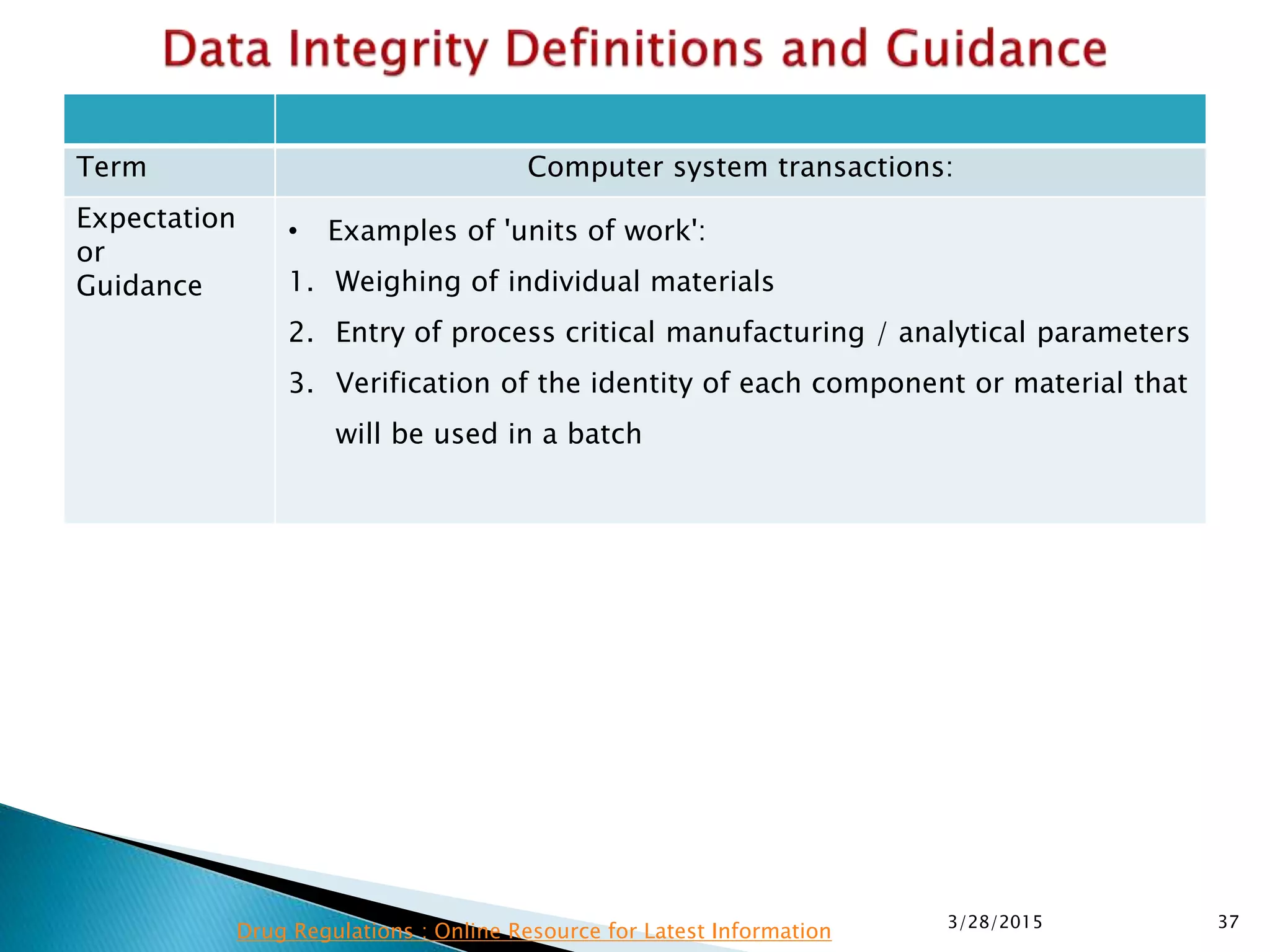
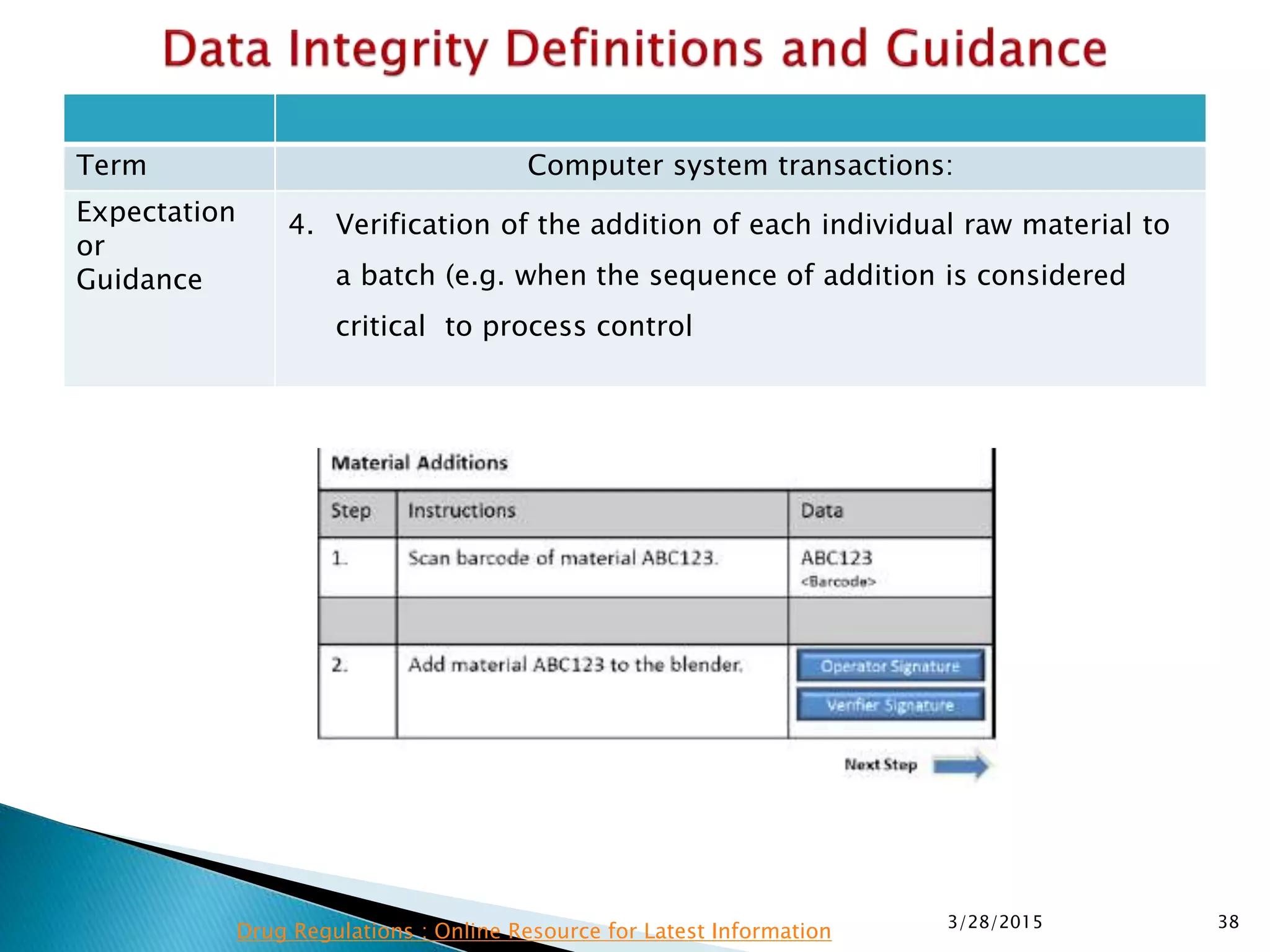
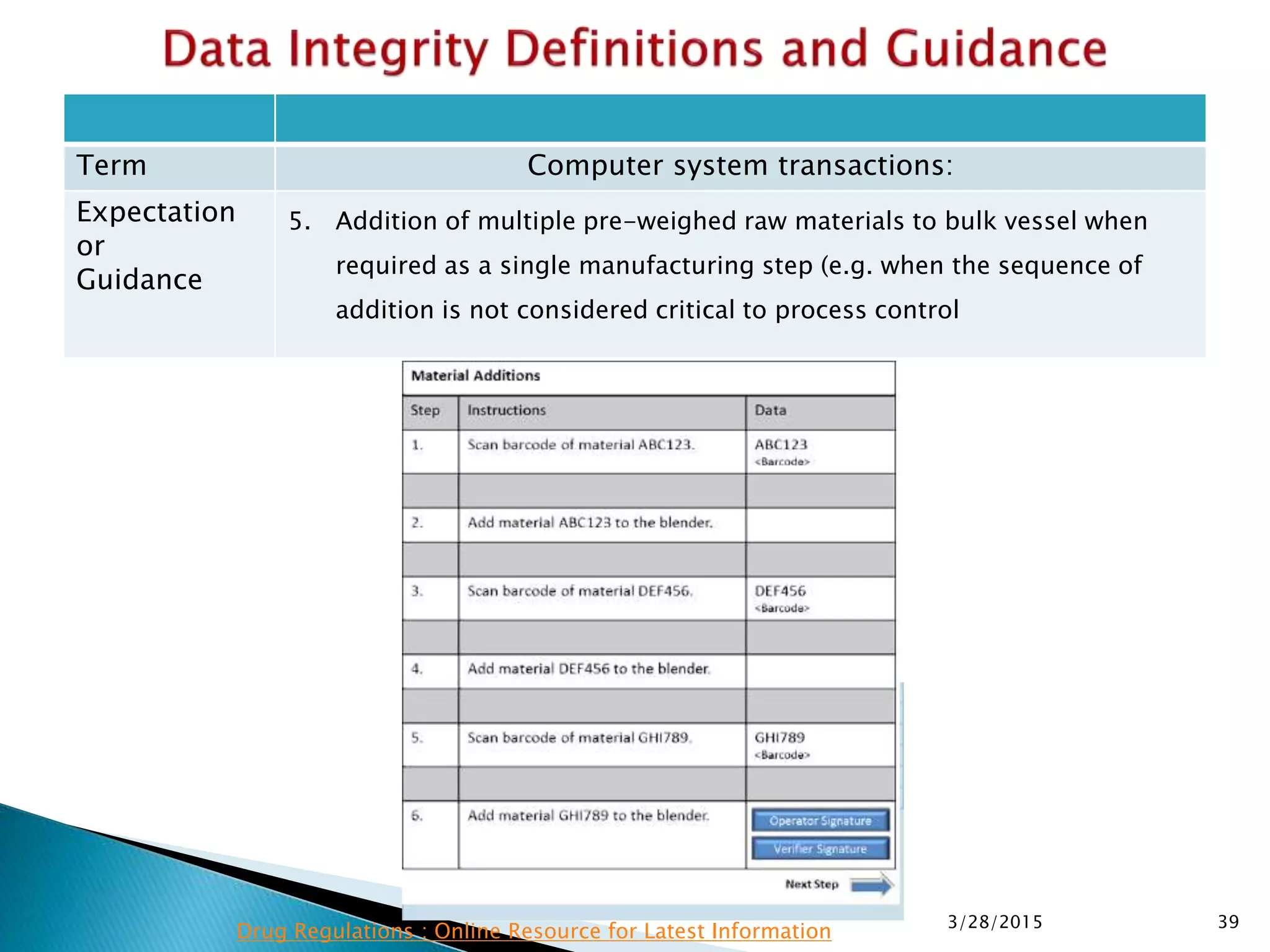
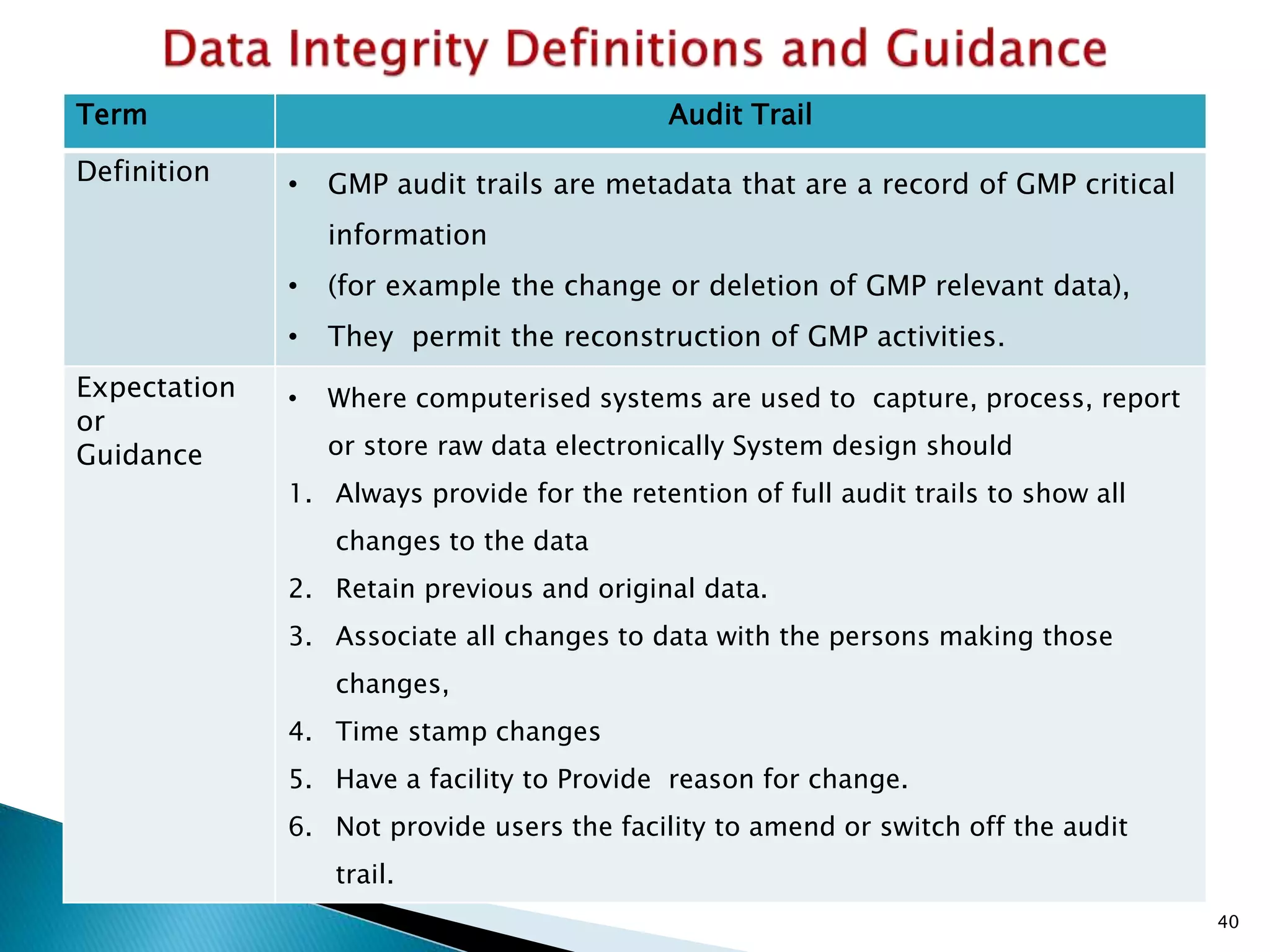
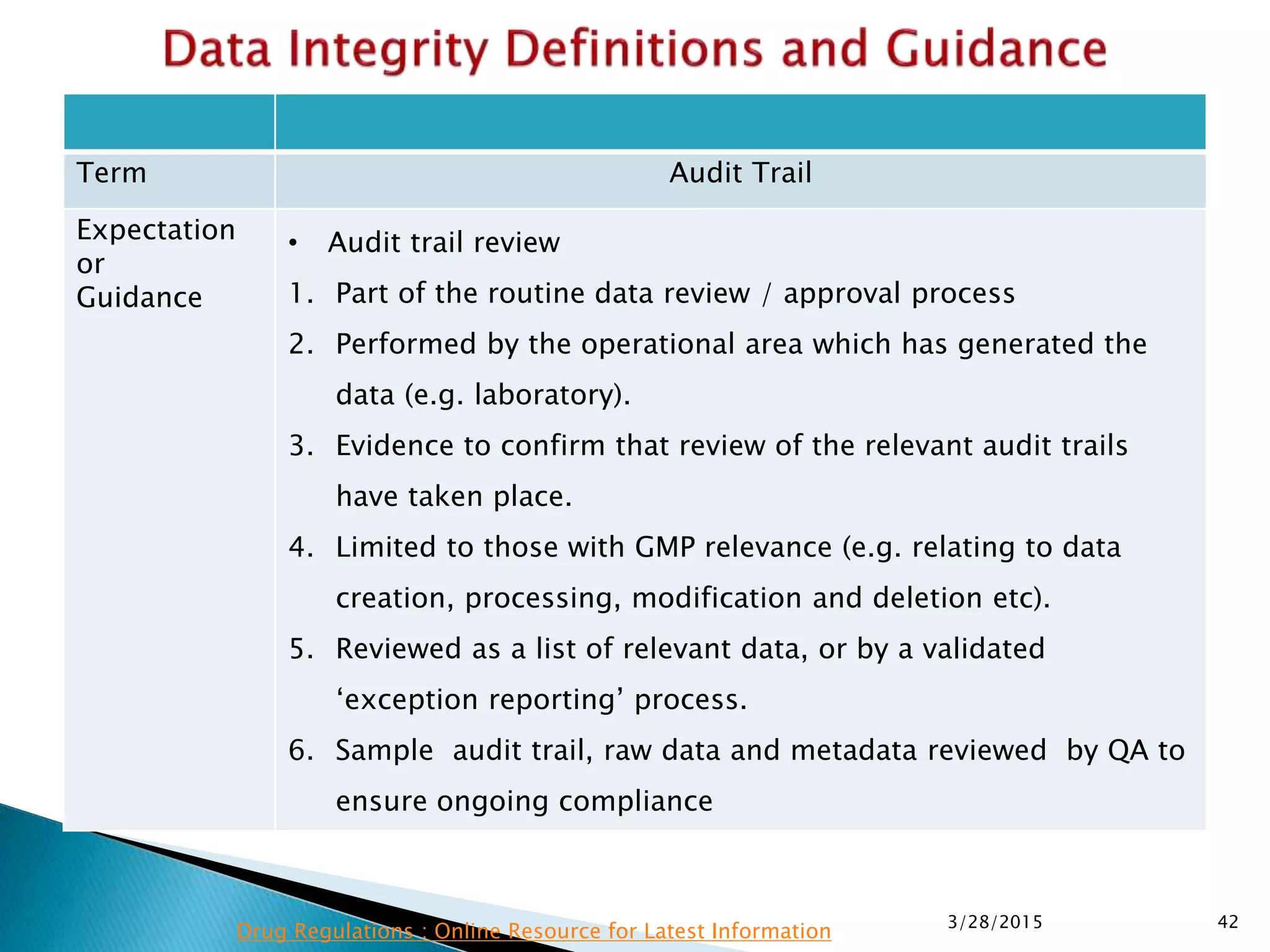
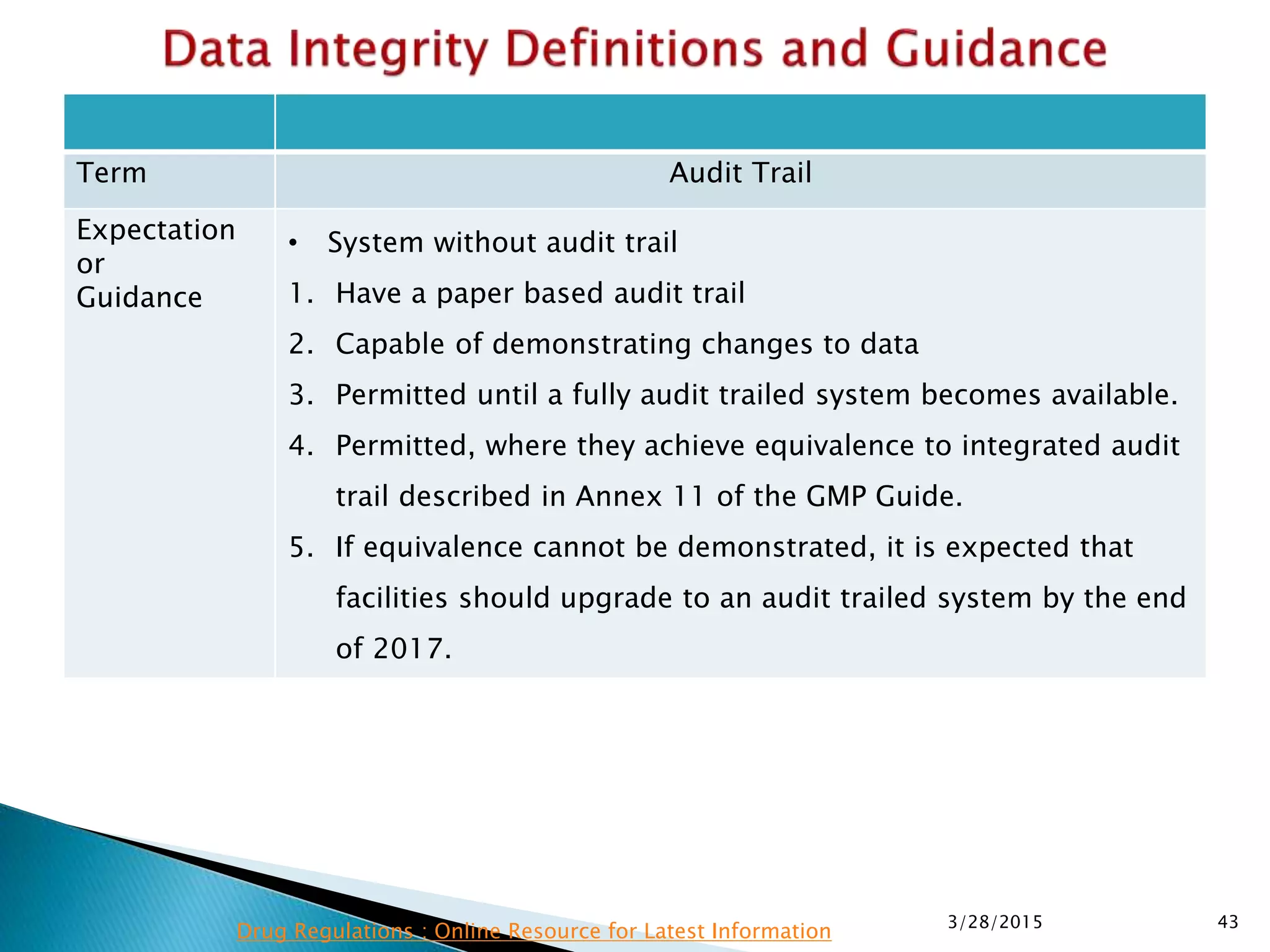
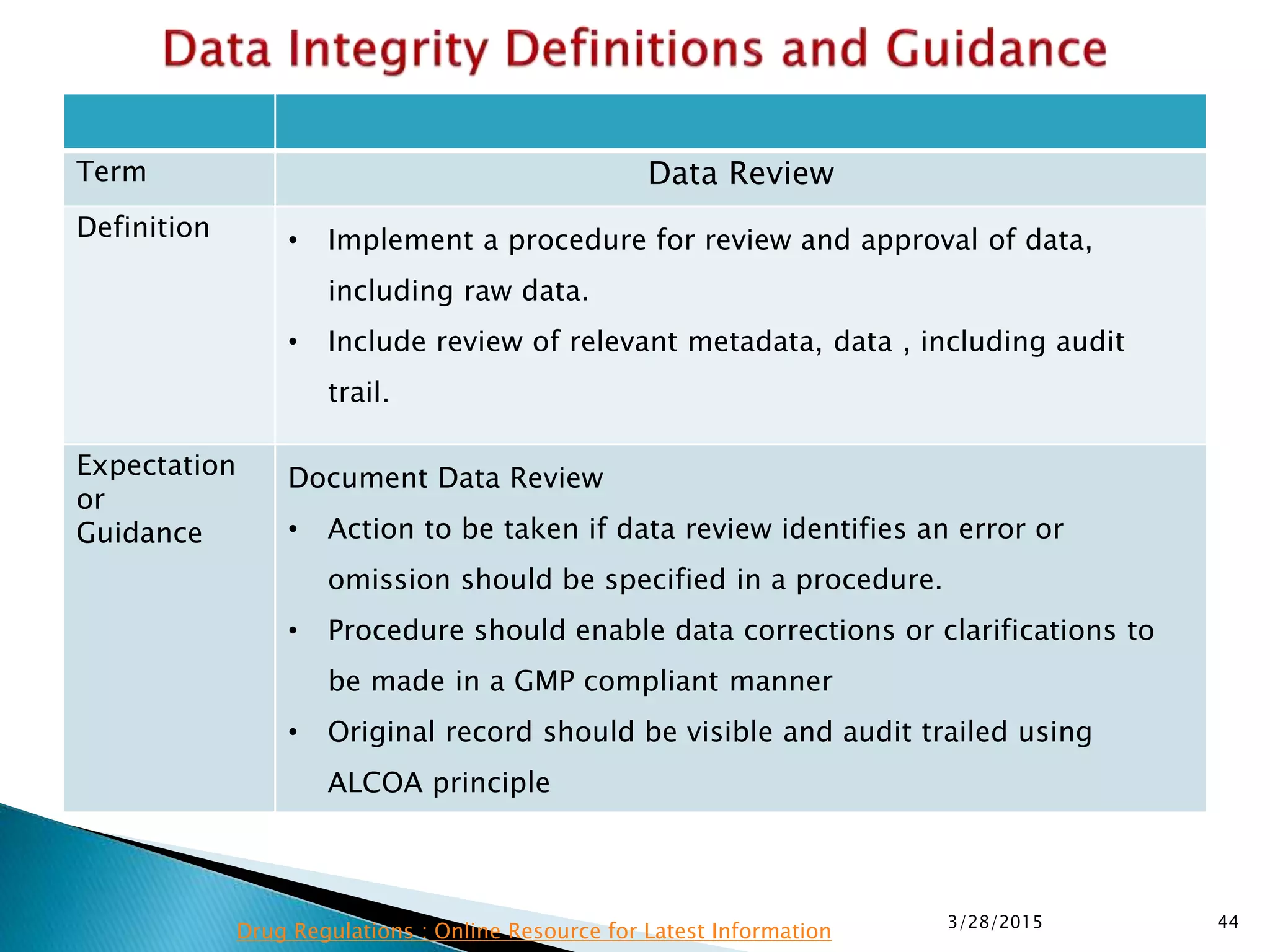
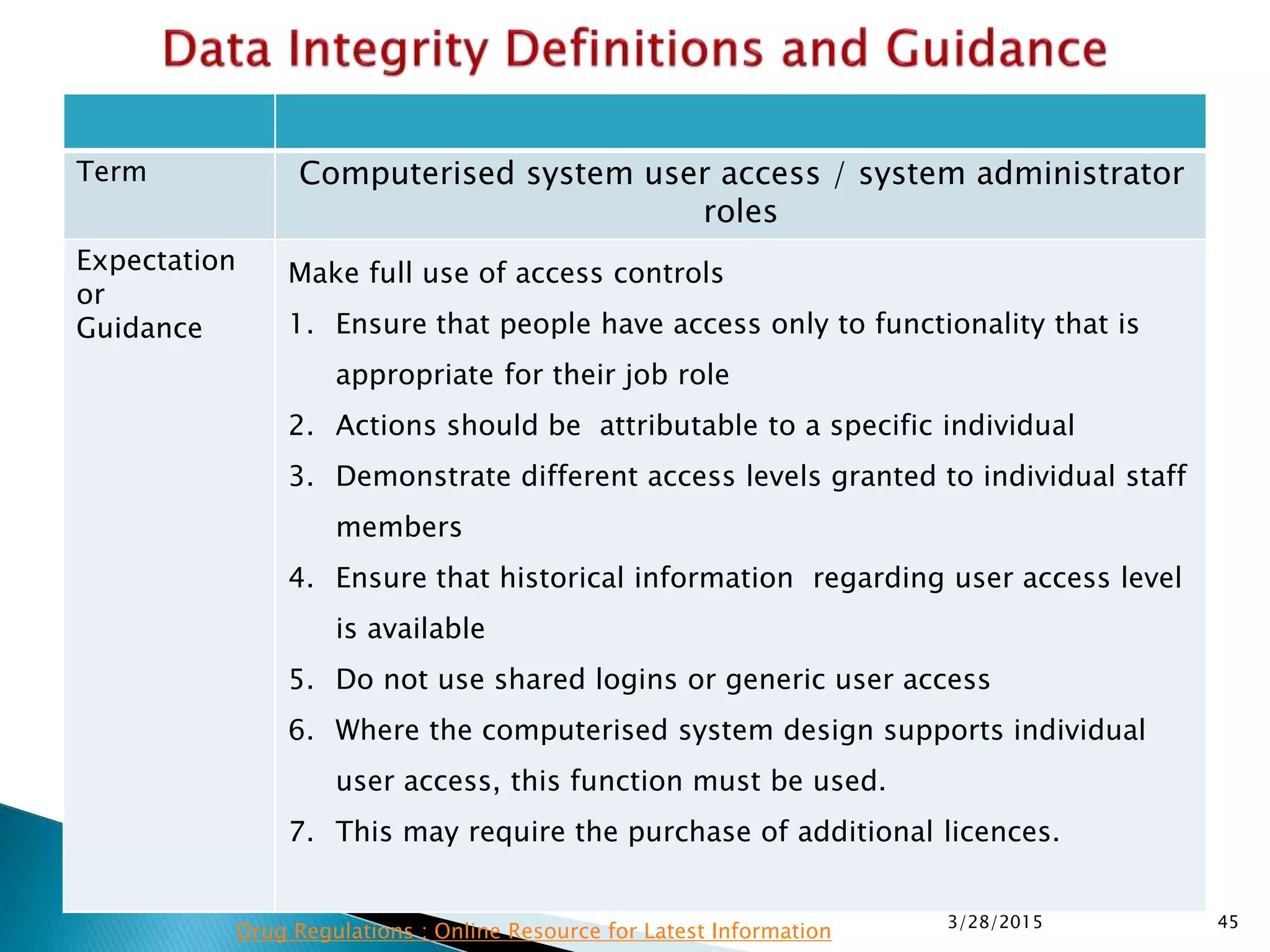
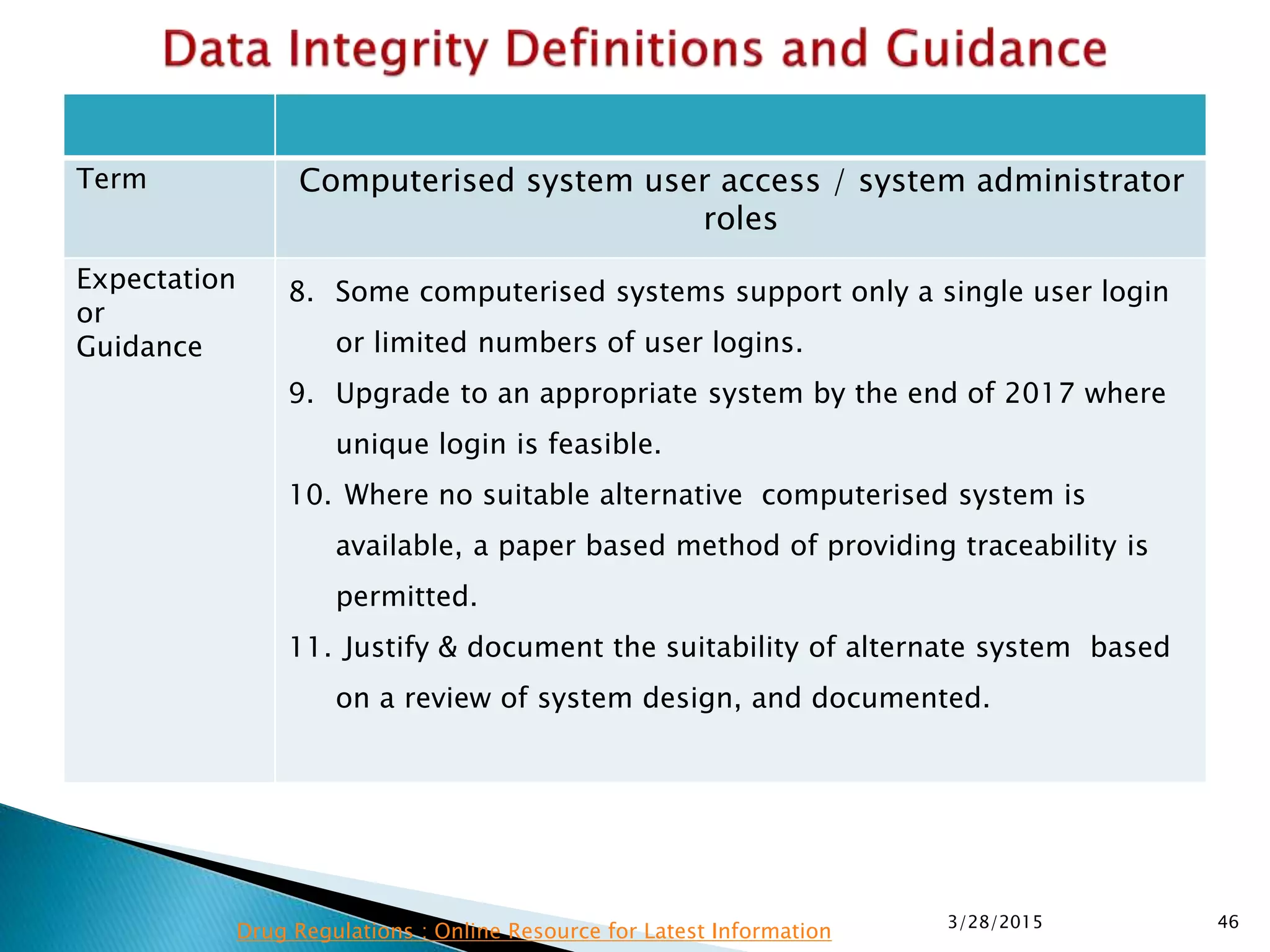
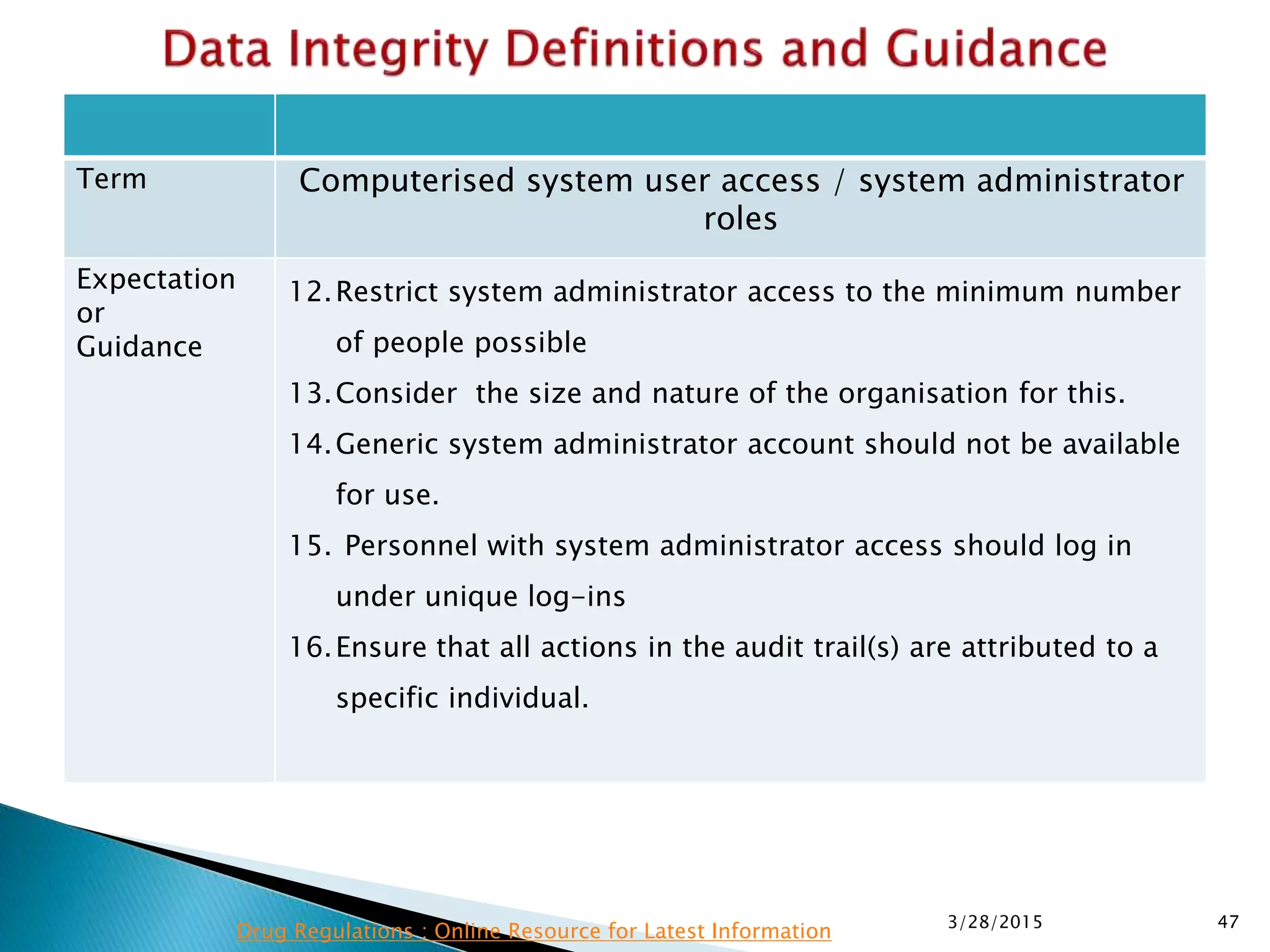
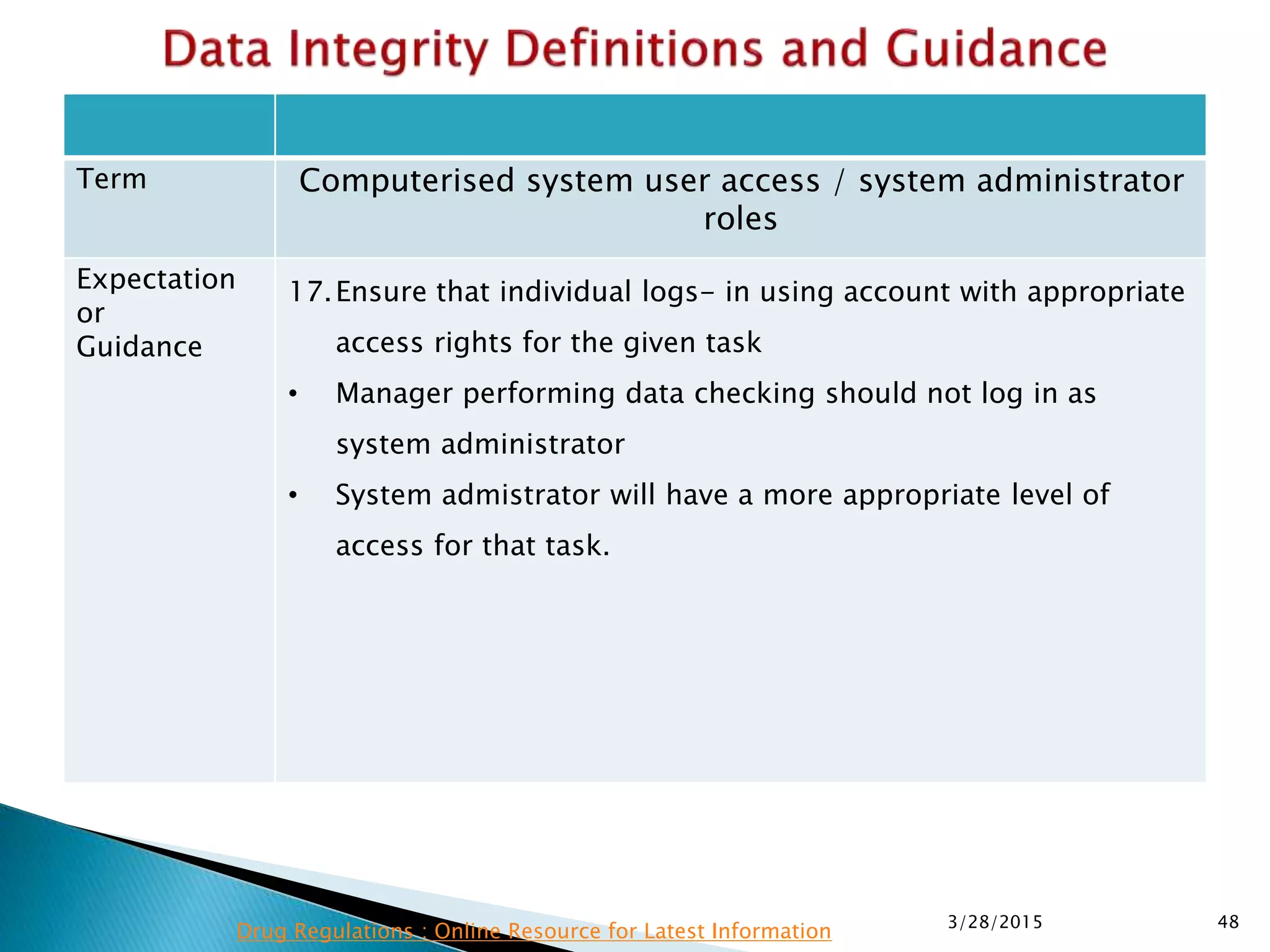
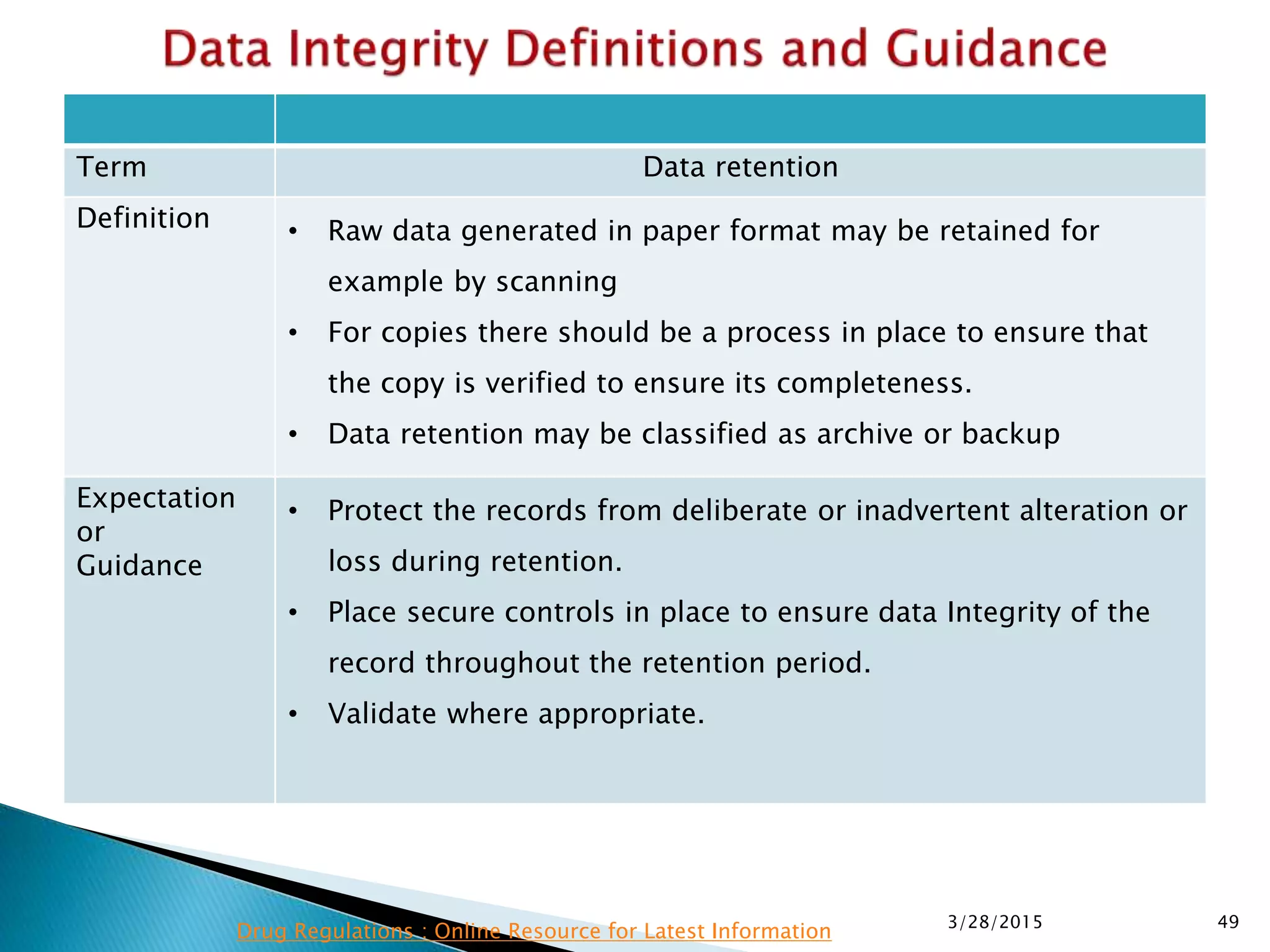
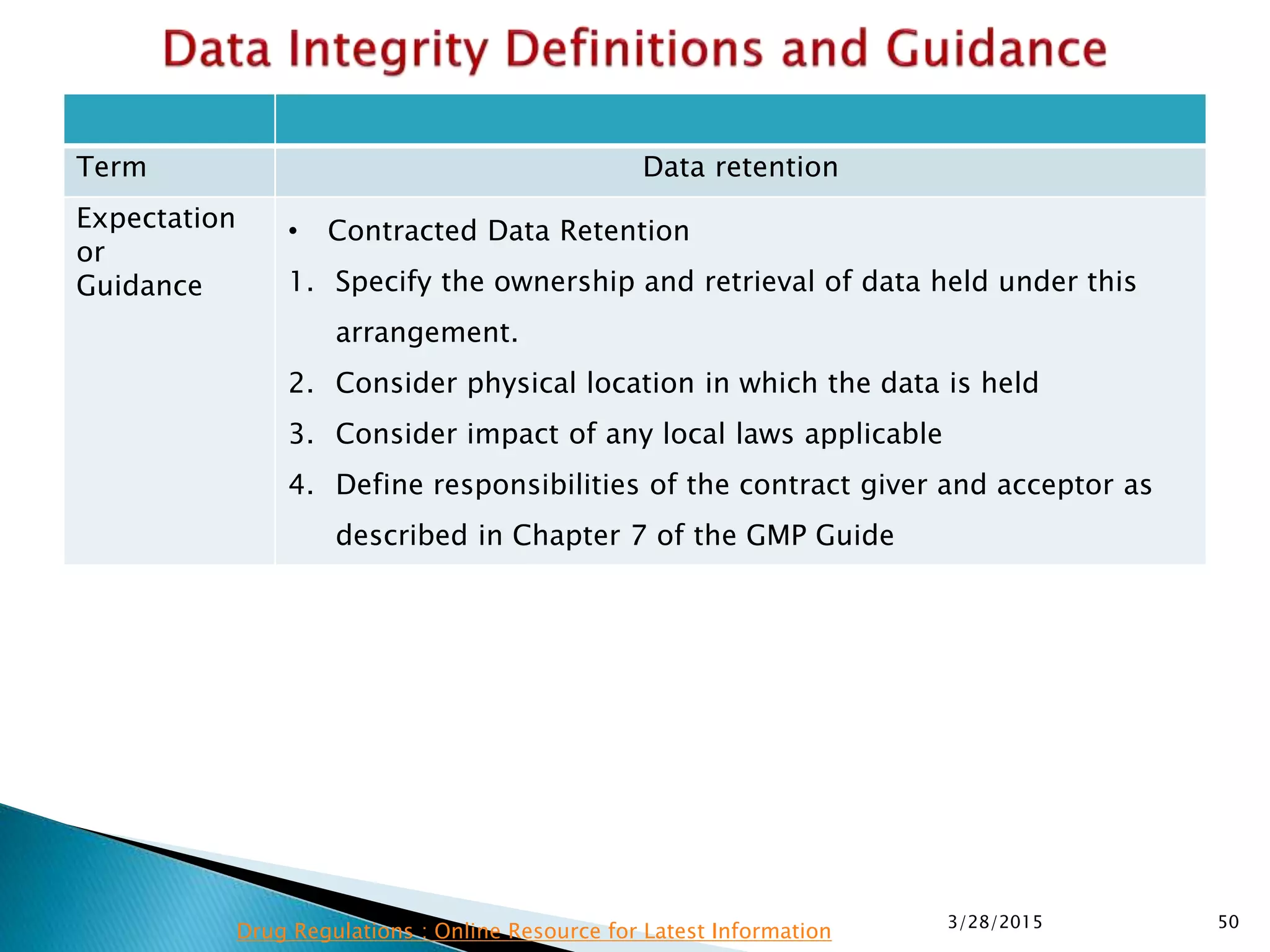
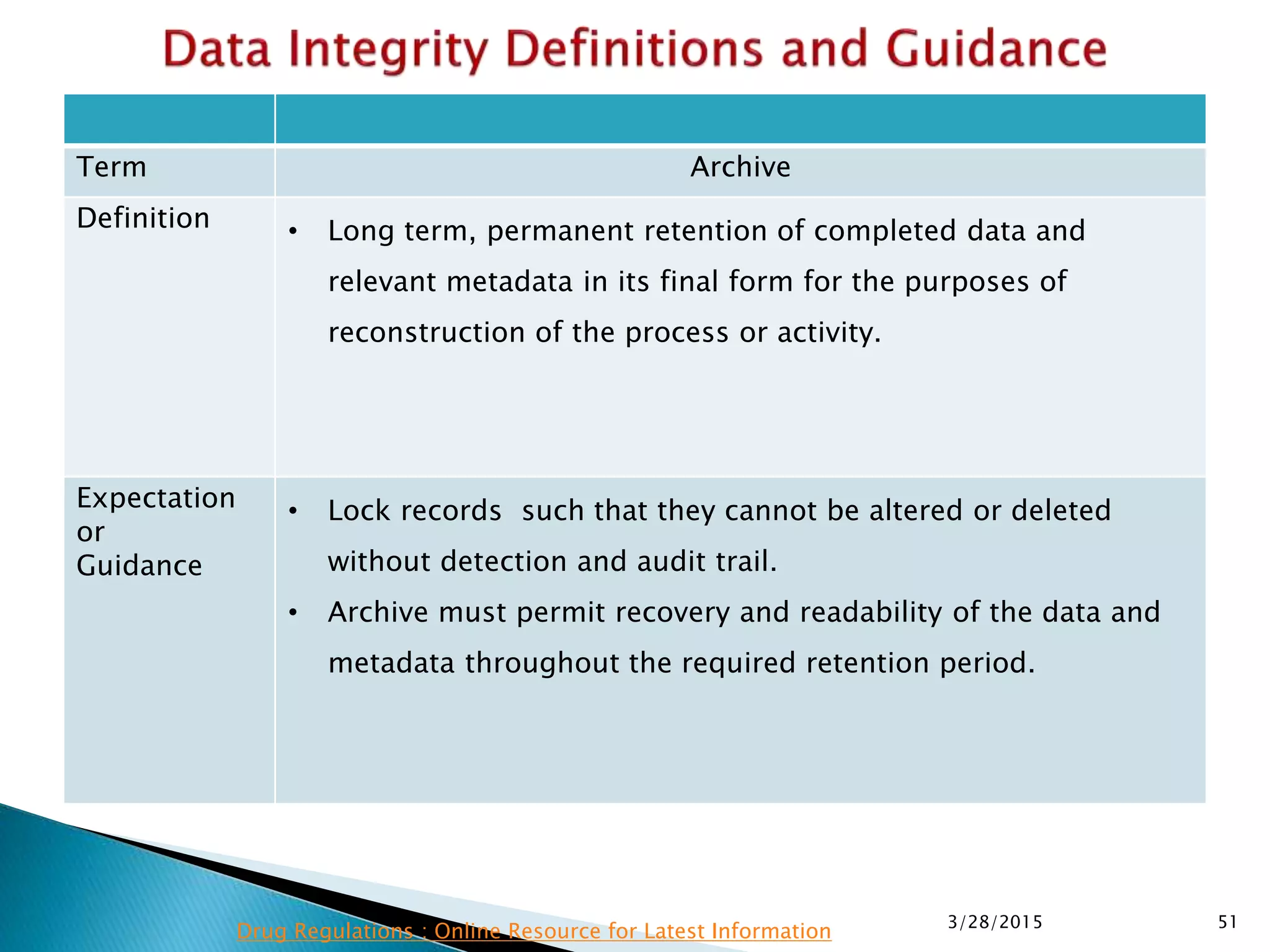
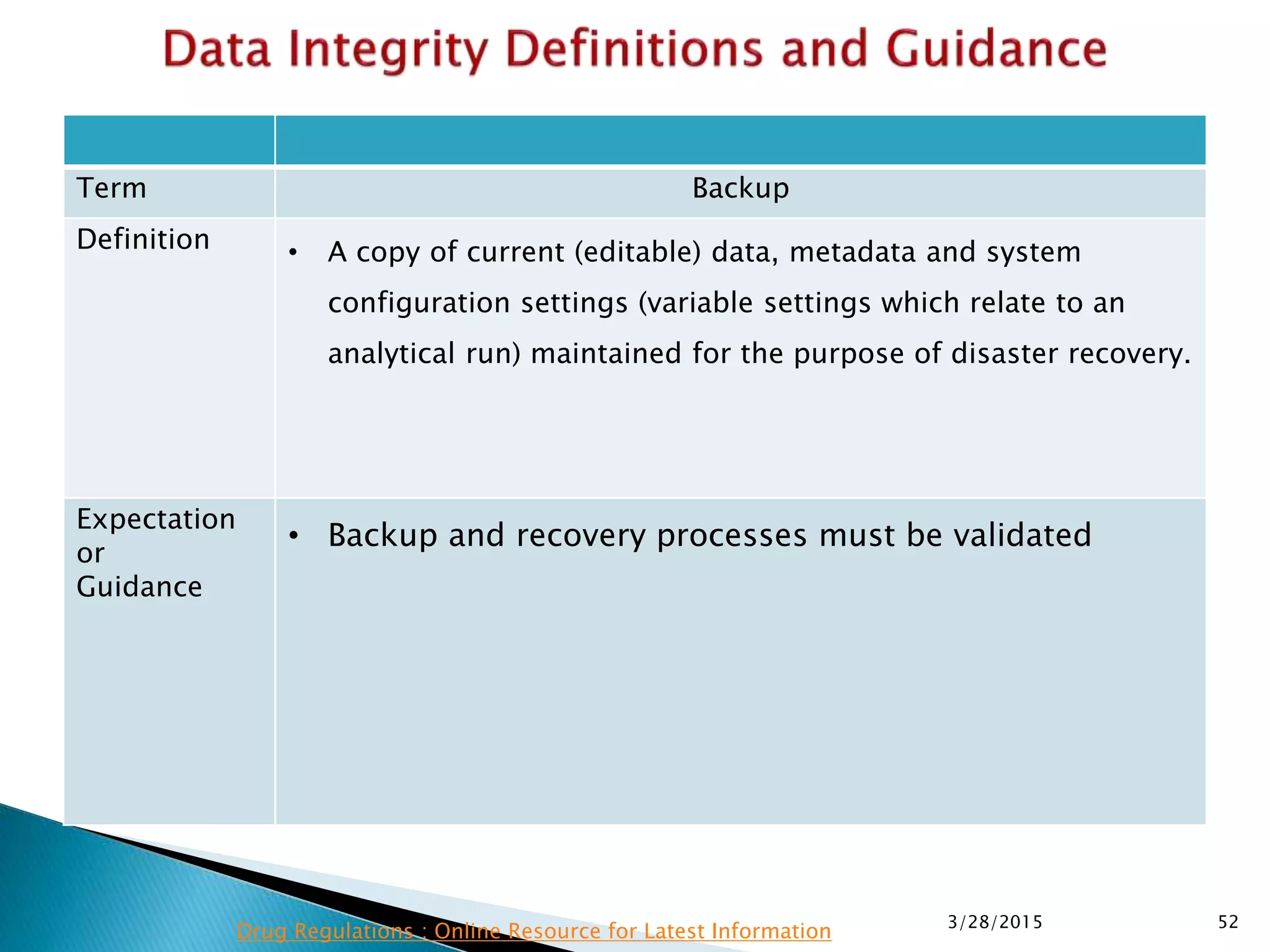
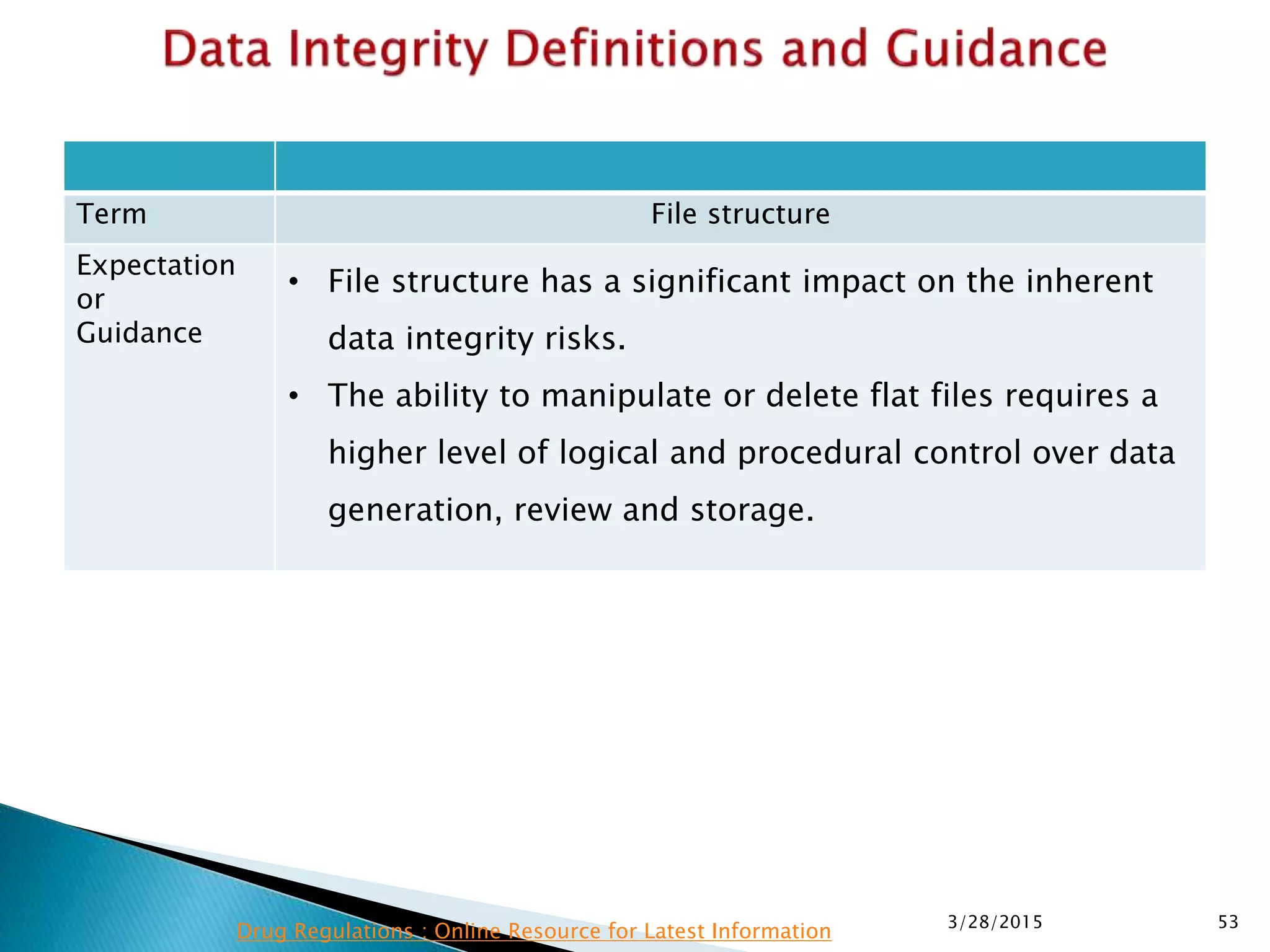
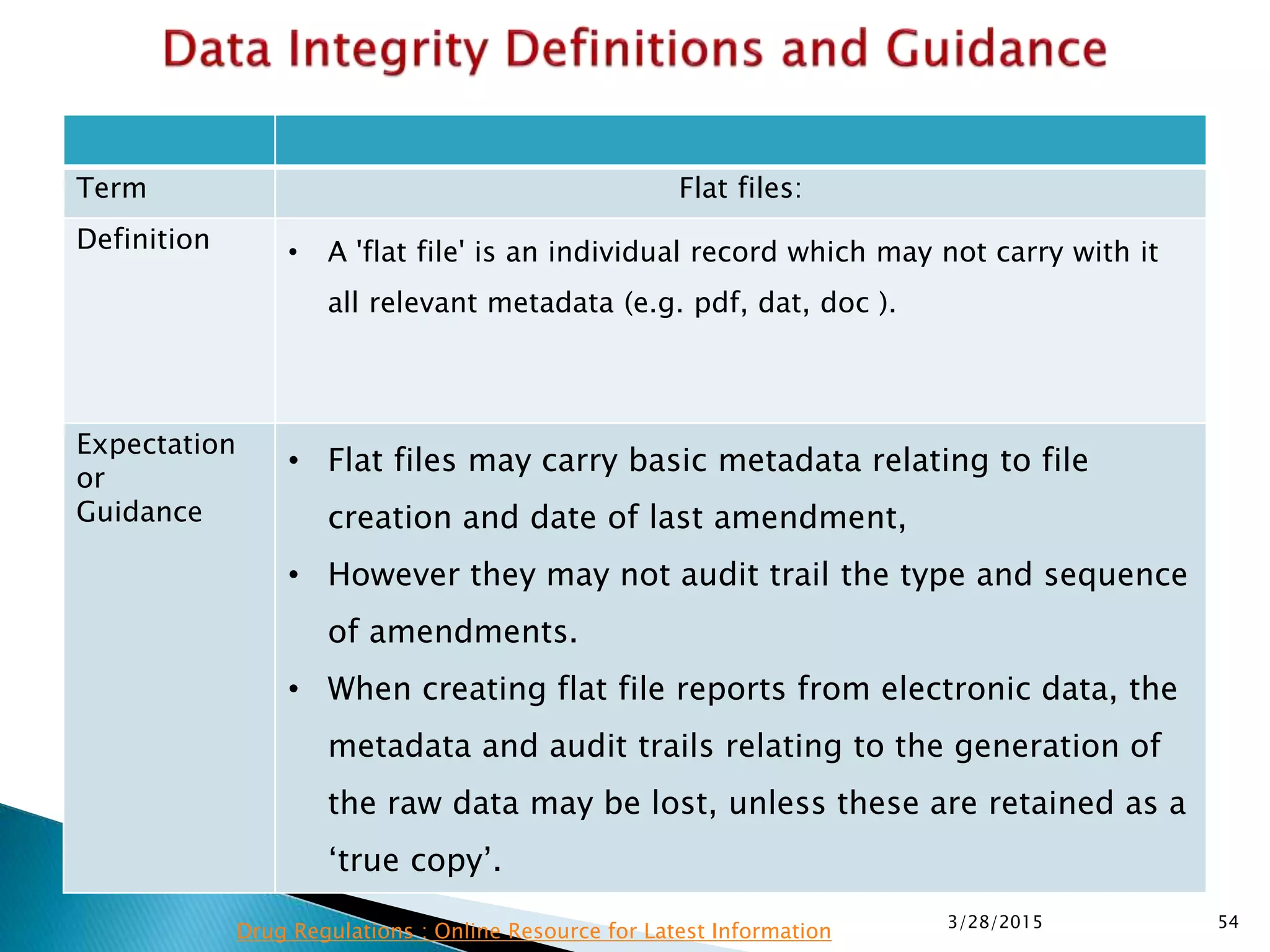
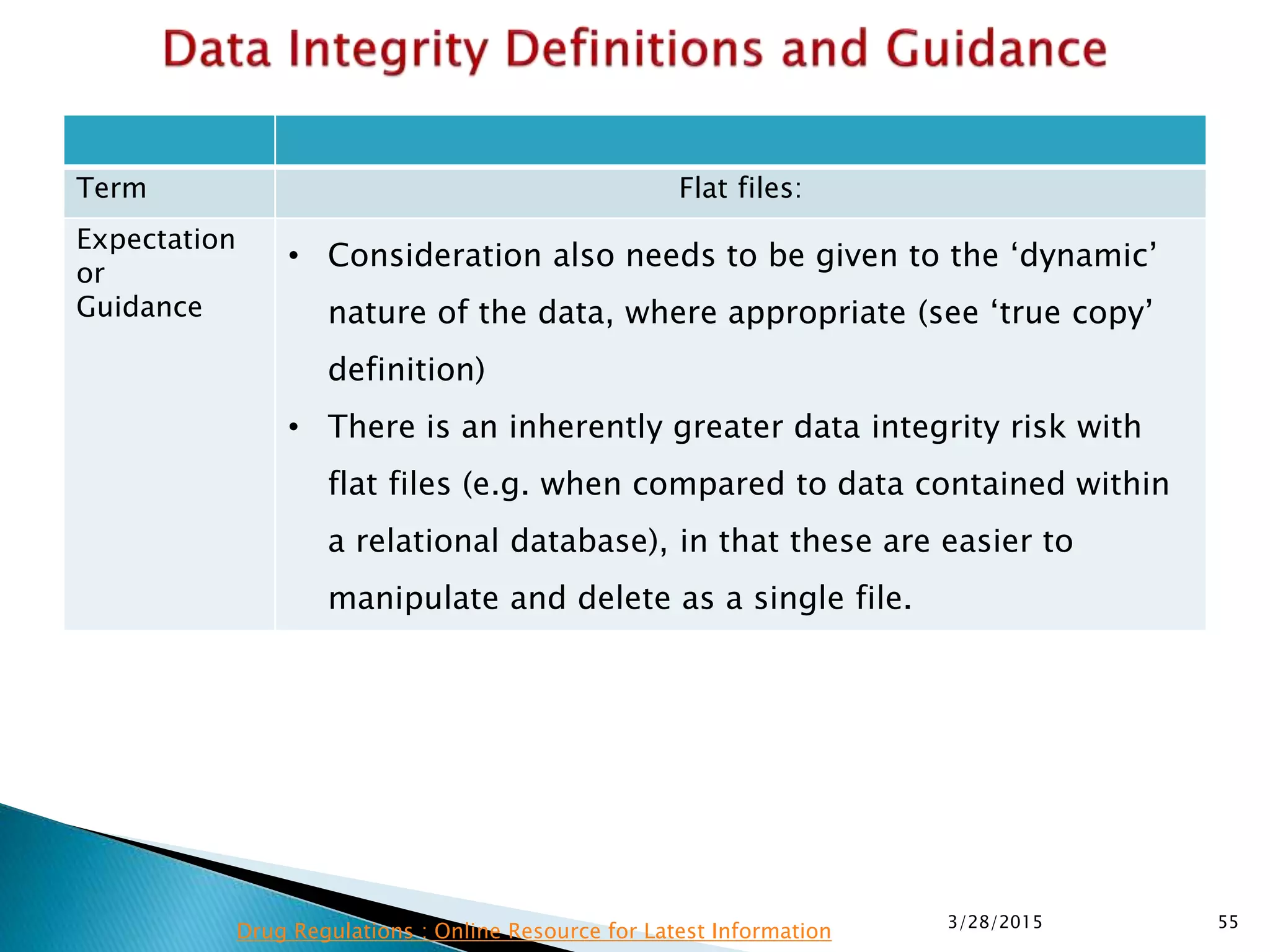
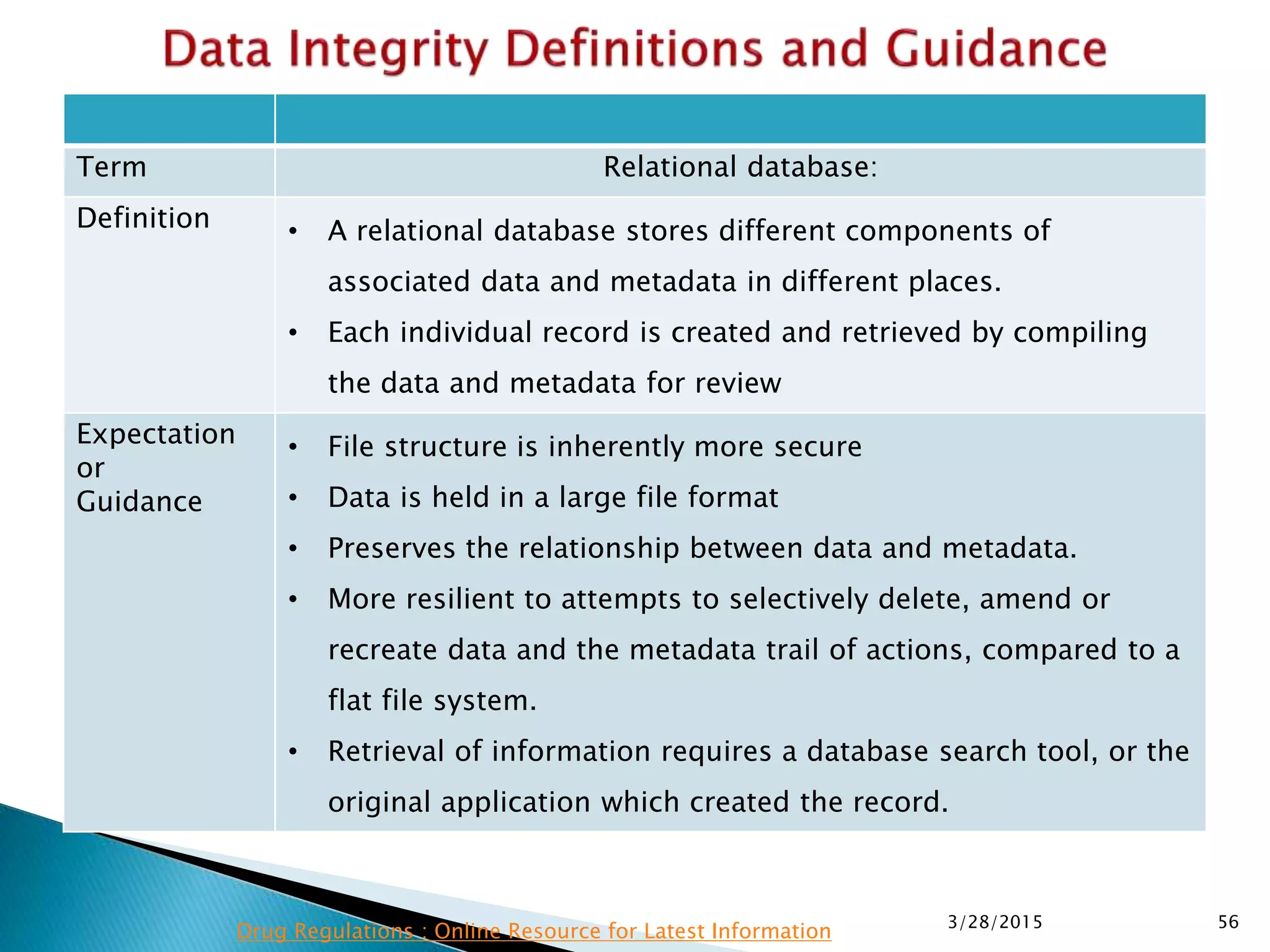
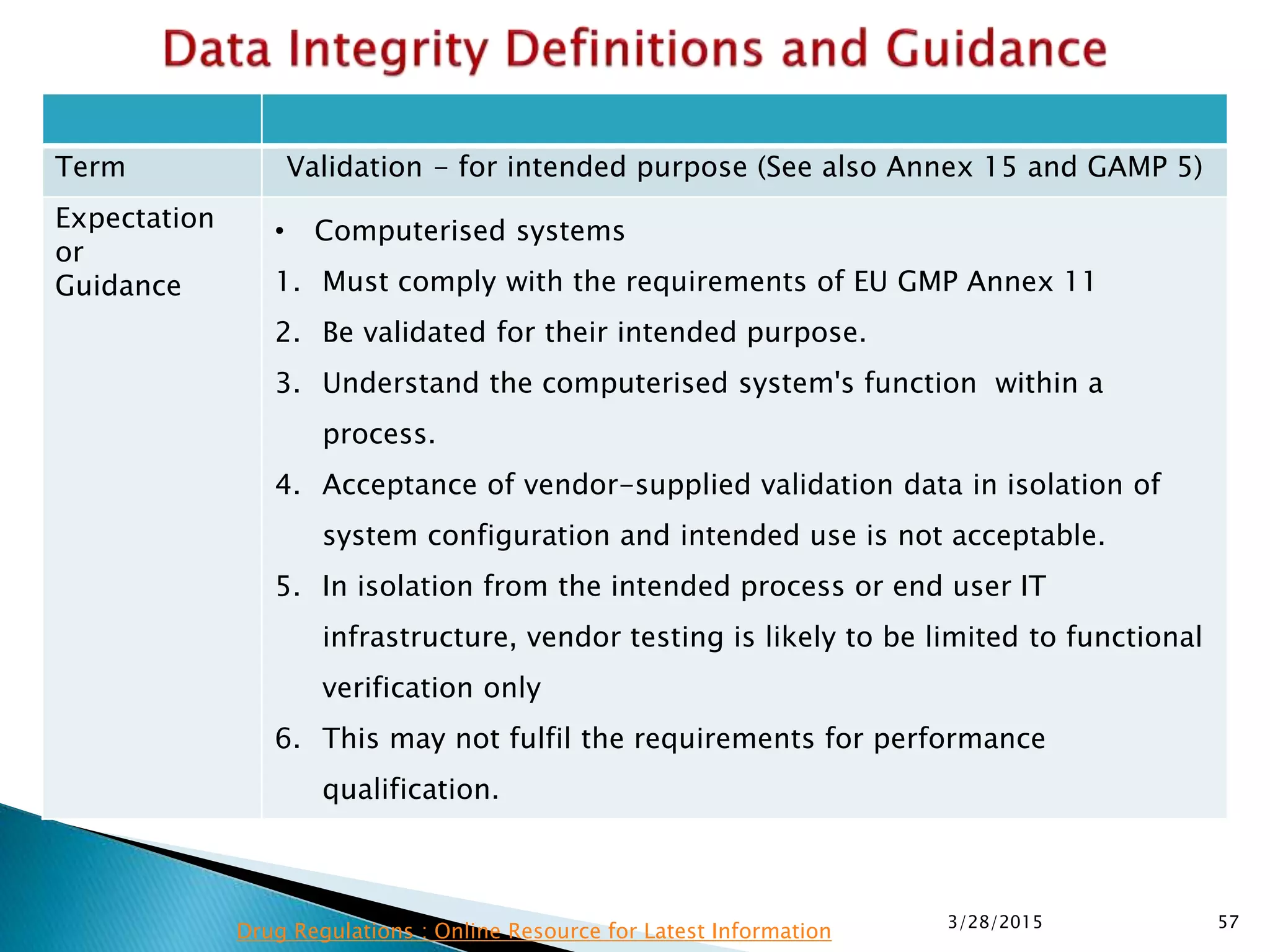
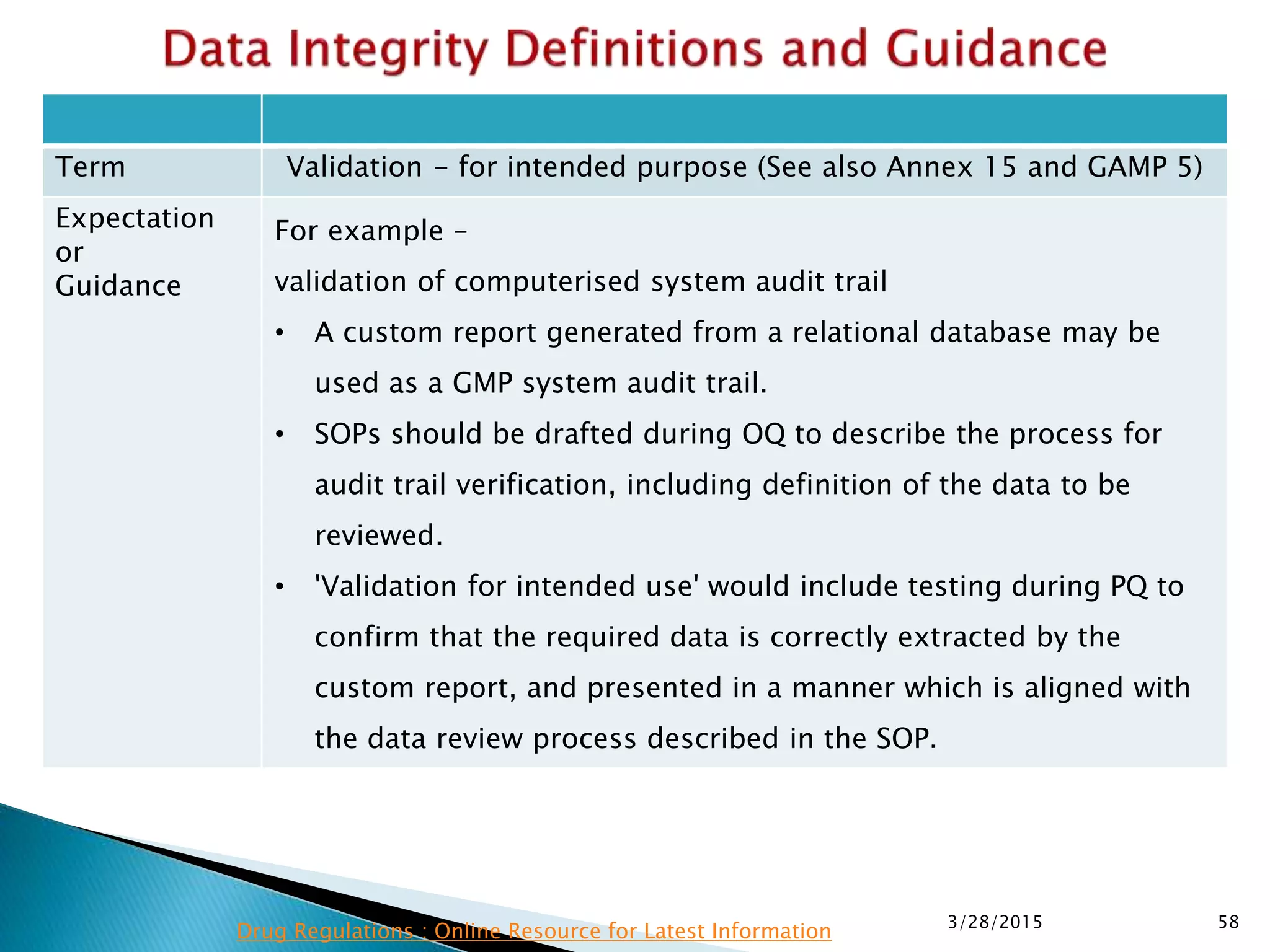
This presentation by the nonprofit organization 'Drug Regulations' provides guidance on data integrity and its critical role in ensuring pharmaceutical quality systems. It outlines expectations from the MHRA concerning both manual and electronic data management, emphasizing the need for adequate data governance, training, and the importance of maintaining accuracy and completeness throughout the data lifecycle. The document also discusses the implications of data integrity failures and the necessary organizational practices to uphold regulatory compliance.