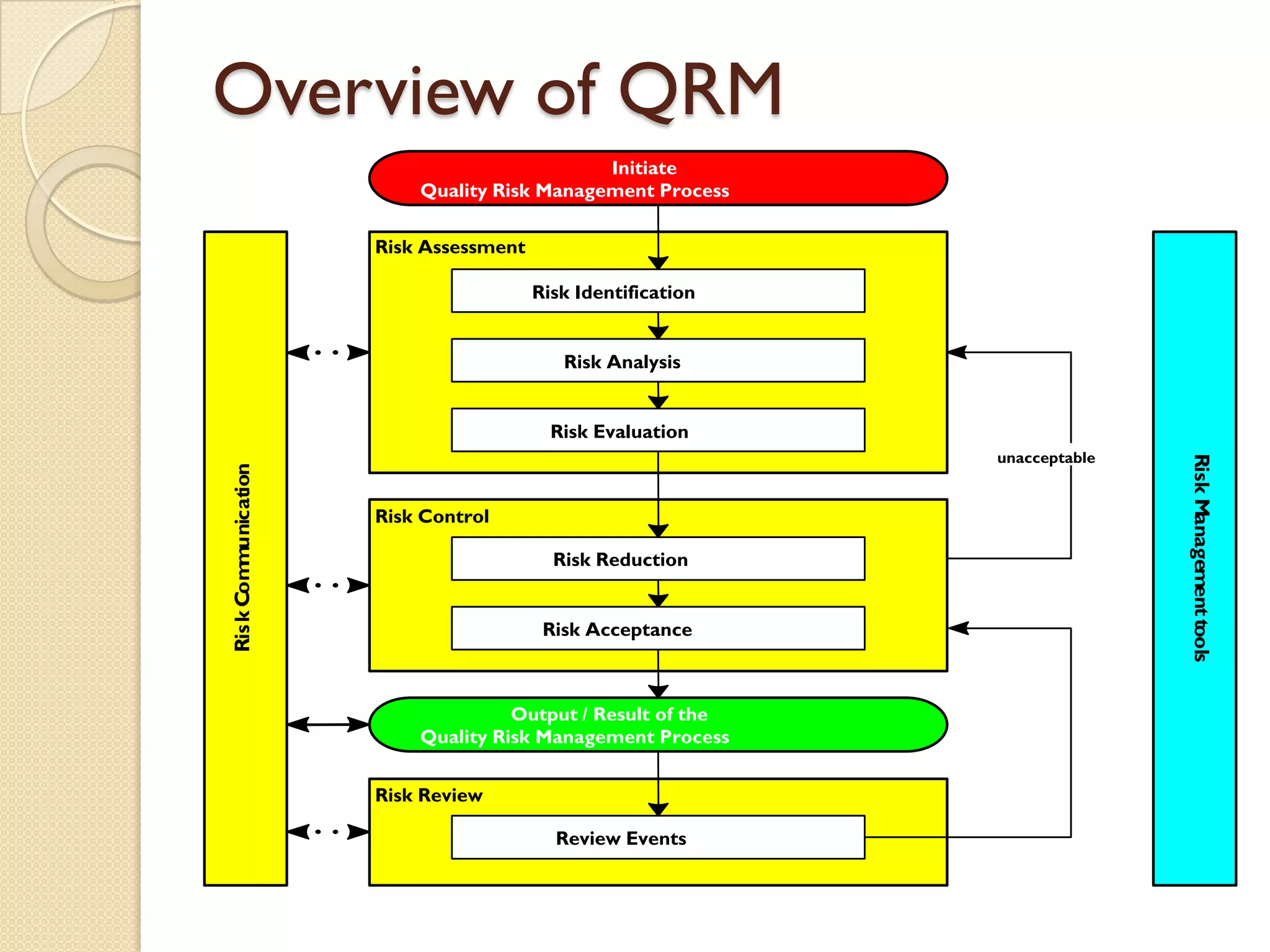
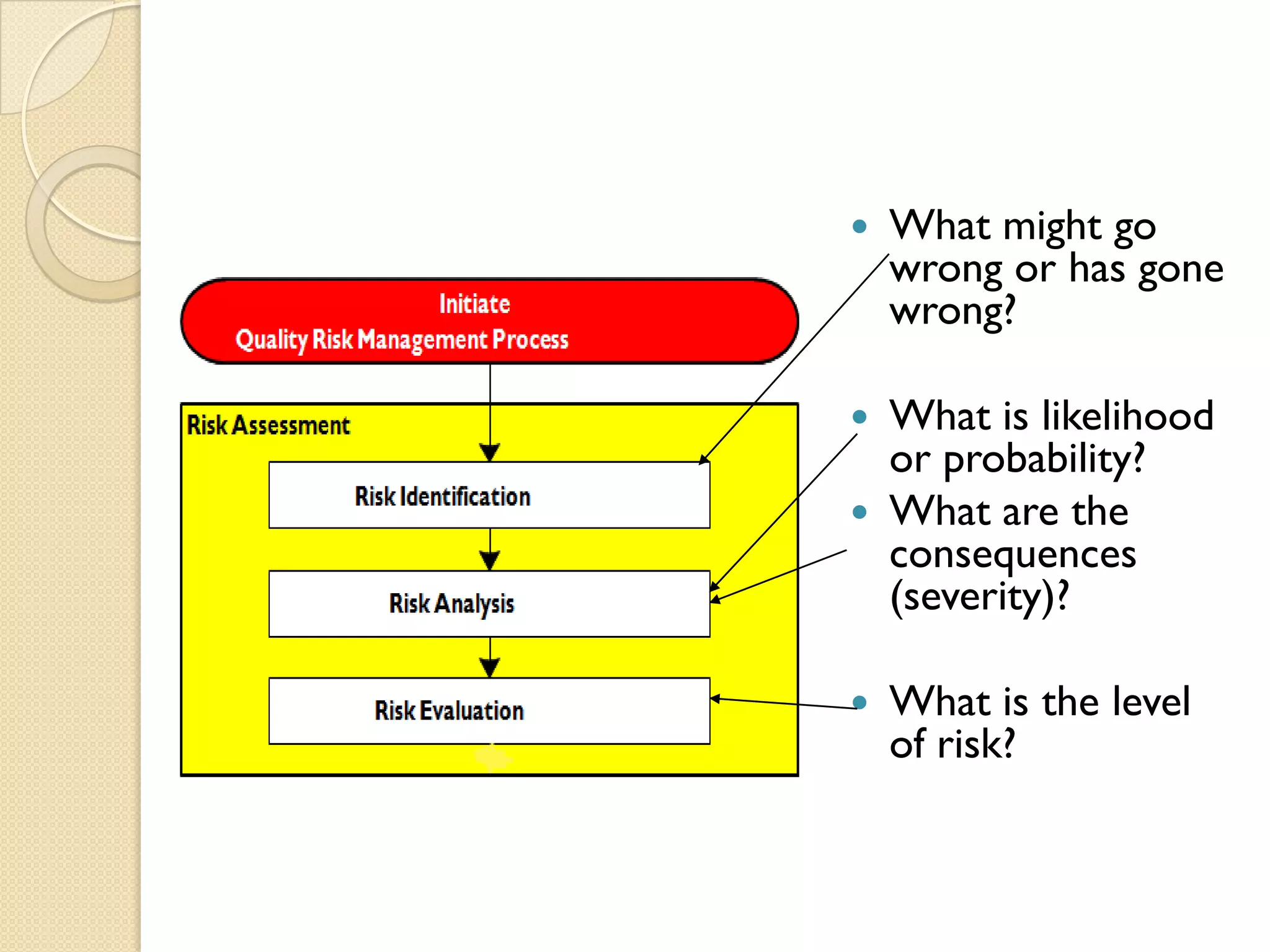
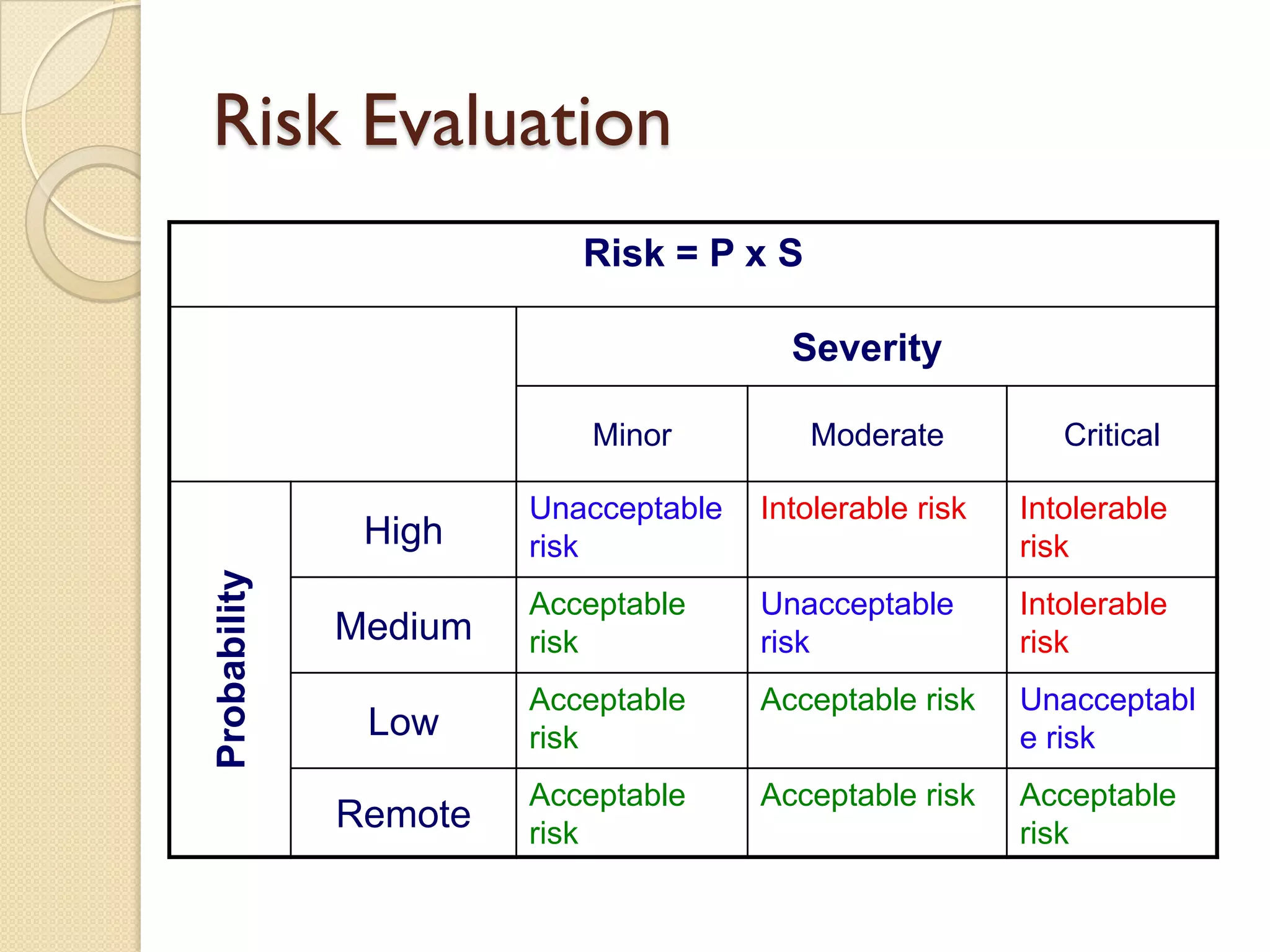
Quality Risk Management (QRM) is a systematic process that assesses, controls, communicates, and reviews risks to the quality of medicinal products throughout their lifecycle, emphasizing scientific knowledge and patient protection. It involves categorizing hazards, evaluating risks based on severity and frequency, and implementing risk management practices to ensure safety and compliance within the pharmaceutical industry. Effective QRM requires interdisciplinary teams to assess and mitigate risks through a structured approach focusing on continuous monitoring and adaptation.