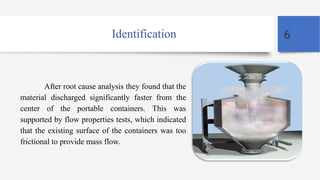
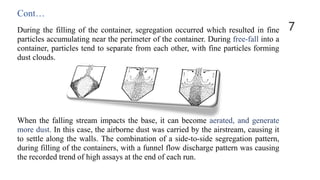
The case study discusses change control in pharmaceutical equipment, emphasizing the importance of maintaining validated status during production. A leading firm faced segregation issues during the validation of a new tablet product, which led to inconsistencies in active ingredient concentration. The solution implemented was the Pharmasok filling system, designed to reduce dusting and segregation by controlling the transfer of mixed powders from blenders to containers.