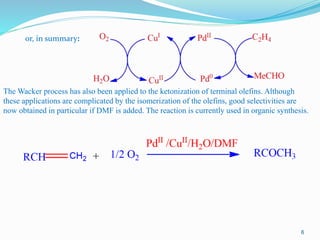
1. The Wacker process oxidizes ethylene to acetaldehyde using a palladium and copper chloride catalyst. Ethylene coordinates to Pd which inserts an oxygen atom and isomerizes to acetaldehyde. CuCl2 helps reoxidize Pd to continue the catalytic cycle.
2. Metal-oxo complexes catalyze many oxidation reactions including allylic oxidation, olefin metathesis, aromatic oxidation, water oxidation, alkene dihydroxylation, and epoxidation of alkenes. These complexes form reactive M=O bonds.
3. Phase-transfer catalysis improves reactions of anionic oxo complexes by using large organic cations to transfer the oxo anion


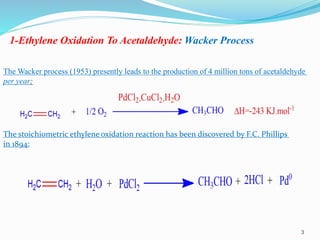
![In aqueous medium, PdCl2 is actually in the form [PdCl4]2–. The detailed mechanism has only
been proposed in 1979 by Bäckwall and Stille the catalytic cycle below). The isomerization of
the hydroxyethyl ligand by β-elimination and re-insertion before decomposition to acetaldehyde
has been demonstrated by the fact that addition of D2O, known to deuterate an enol, does not lead
to the incorporation of deuterium in acetaldehyde. The rate law is:
It can be deduced that the rate-limiting step involves, in its transition state, a palladium complex
containing an ethylene molecule and having lost two chloride ligands and a proton.
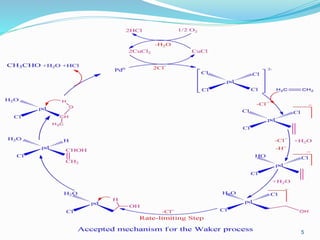
In this stoichiometric reaction, the palladium metal precipitates. In the presence of oxygen, the
thermodynamics is favorable to the re-oxidation of Pd0 to PdII. The structural transformation
required for Pd oxidation slows down this re-oxidation, however. The Pd0 colloid formation is
thus faster and the kinetics is unfavorable for catalysis. It is the introduction of CuCl2 as a
cocatalyst that allowed to make this process catalytic. Indeed, CuCl2 can rapidly re-oxidize Pd0 to
PdCl2 because of fast inner-sphere Cl transfer via bridging Cu and Pd. CuCl formed can be
oxidized by O2. The redox CuI/CuII system works as a redox catalyst in a way very similar to
that of biological systems. The coupling between coordination catalysis and redox catalysis is
thus a biomimetic concept. The overall catalytic cycle follows](https://image.slidesharecdn.com/chapter22-150425112654-conversion-gate01/85/OXIDATION-OF-OLEFINS-4-320.jpg)
![2-Hydroxylation By Metal-Oxo Complexes
2.1Metal-Oxo Complexes In Oxidation Catalysis
The complexes with M=O bonds have very different reactivities depending on the nature of the
transition metal M. The early transition metals are very oxophilic and form M=O bonds that are
not very reactive. These compounds are called oxides. On the other hand, late transition metals
form labile M=O bonds because of the repulsion between the filled d metal orbitals and p oxygen
orbitals. They are called metal-oxo complexes. The metal-oxo complexes can form and
regenerate by transfer of an oxygen atom onto a transition metal using an oxygen atom donor
such as H2O2 or from O2 by double oxidative addition giving a metal-dioxo complex. They play
an essential role in oxidation catalysis. They can also, as oxidants, remove one electron from an
oxidizable substrate (for instance, in the case of [MnO4]– for alkylated aromatics). There are
many binary mono- and polymetallic complexes, i.e. containing only one type of metal and the
oxo ligands. There are also many compounds containing one or several oxo ligands in addition to
other ligands. There are many oxidation reactions that are catalyzed by metal-oxo complexes as
illustrated by the non-exhaustive following table.
7](https://image.slidesharecdn.com/chapter22-150425112654-conversion-gate01/85/OXIDATION-OF-OLEFINS-7-320.jpg)
![Summary Of Oxidation Of Olefins
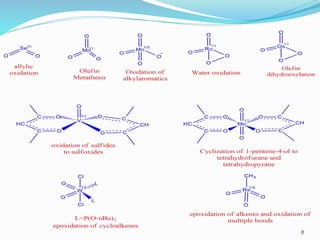
1-Oxidation of ethylene to acetaldehyde (Wacker process)
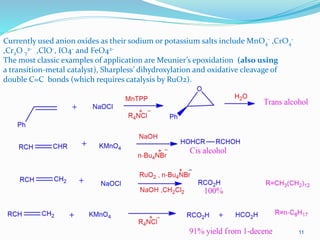
2-The various metal-oxo complexes catalyze numerous reactions:
allylic oxidation (SeO2), olefin metathesis (MoO3), aromatic oxidation (MnO4
–), water oxidation
(RuO4), alkene dihydroxylation (OsO4), oxidation of sulfides to sulfoxides ([VO(acac)2]),
epoxidation of alkenes (WO2ClL2 or ReO3Me) and cyclization of 1-pentene-4-ol to THF and THP
([MoO2(acac)2]).
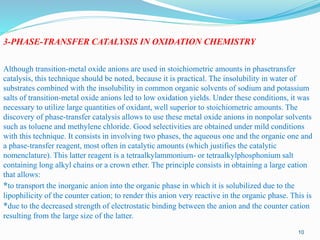
These oxidation reactions are considerably improved by using phase-transfer catalysis (PTC)
when the catalyst is an anionic oxo complex. PTC enhances the reactivity of transition-metal
oxide anions by the introduction of a large organic cation such as R4N+ (with long alkyl arms
for R). This organic cation, the phase-transfer catalyst, carries the oxo-anion from the aqueous
phase into the organic phase and renders it very reactive by decreasing the electrostatic binding
within the ion pair due to its large size.
13](https://image.slidesharecdn.com/chapter22-150425112654-conversion-gate01/85/OXIDATION-OF-OLEFINS-13-320.jpg)
