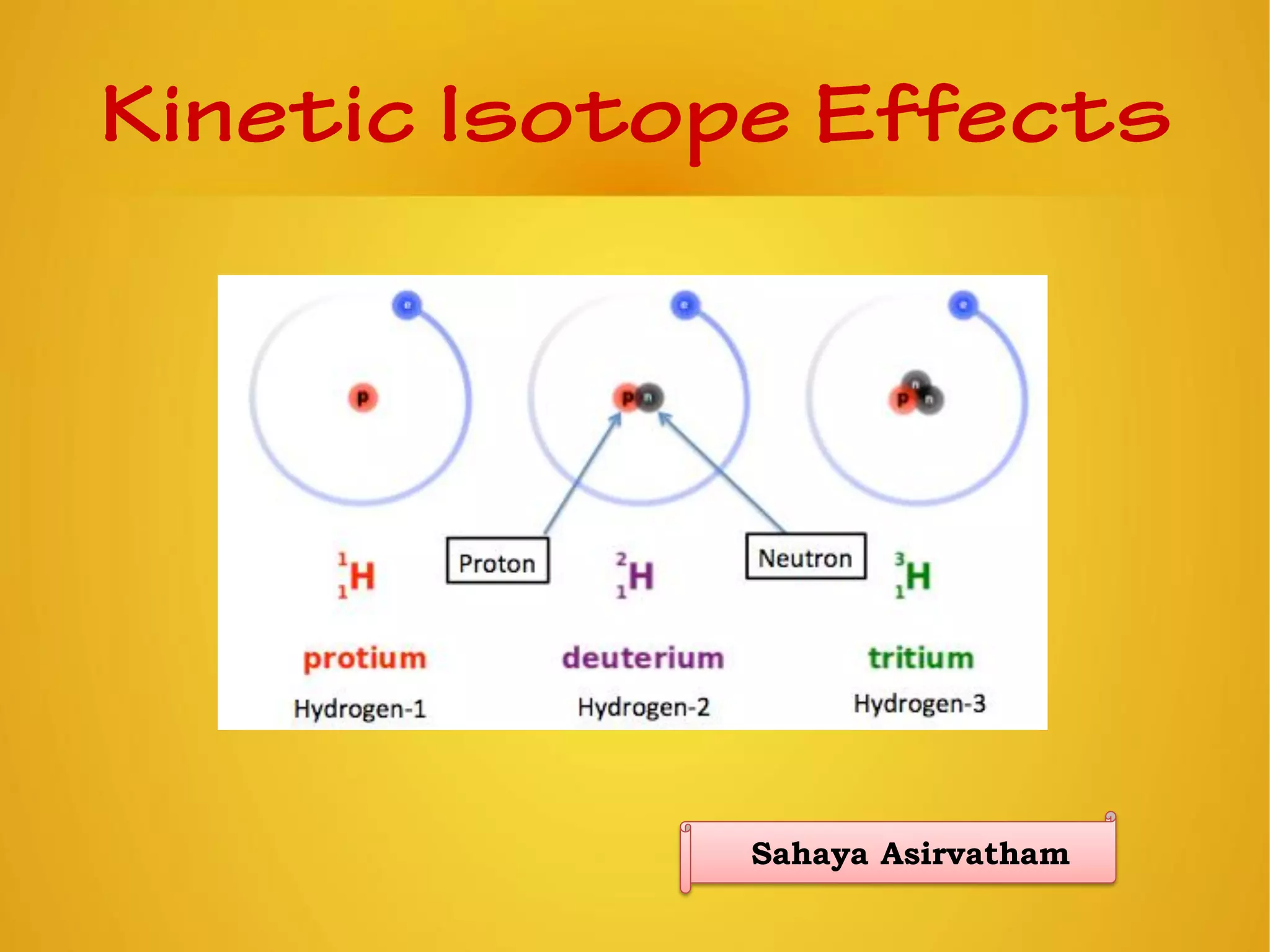
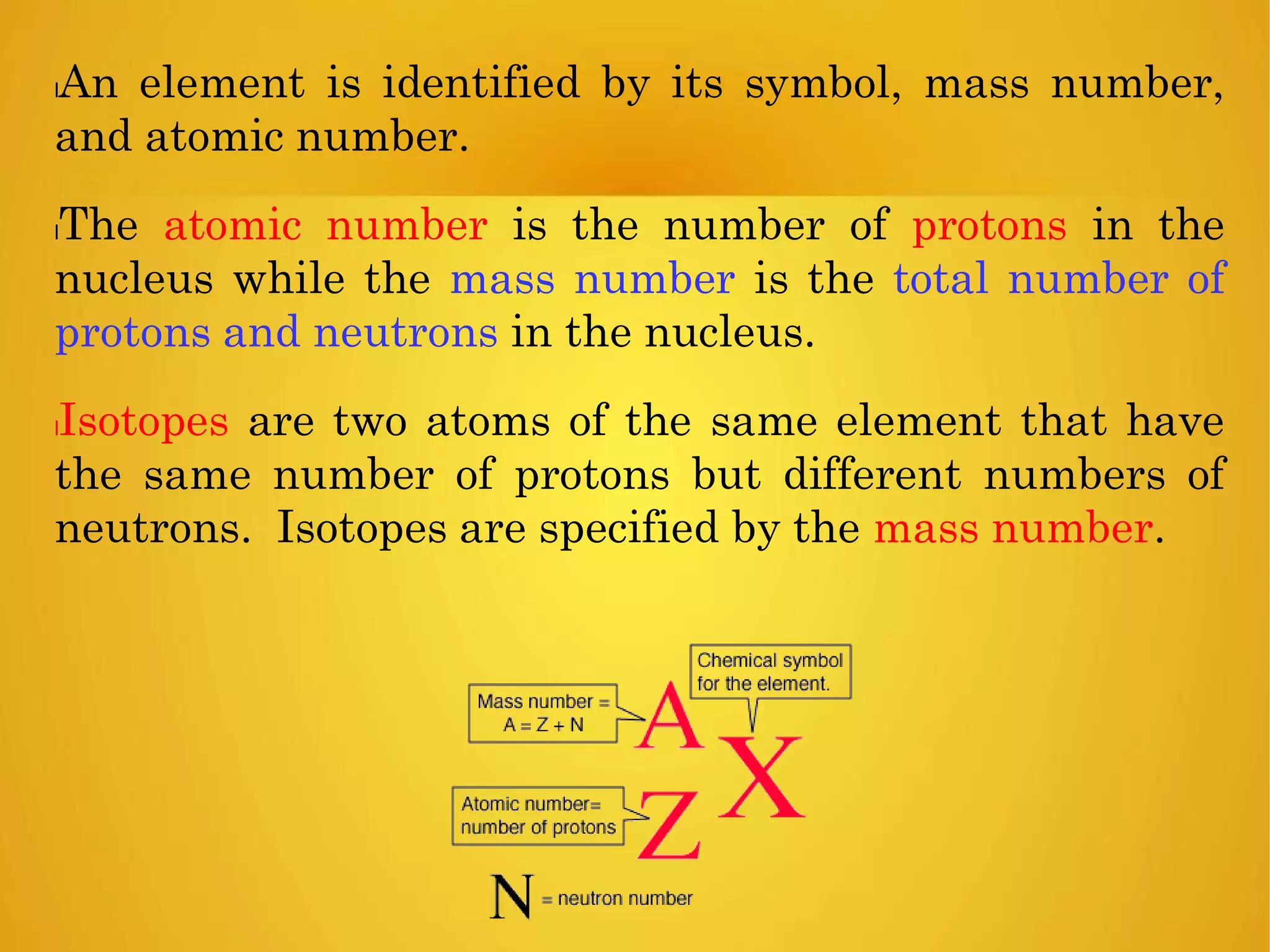
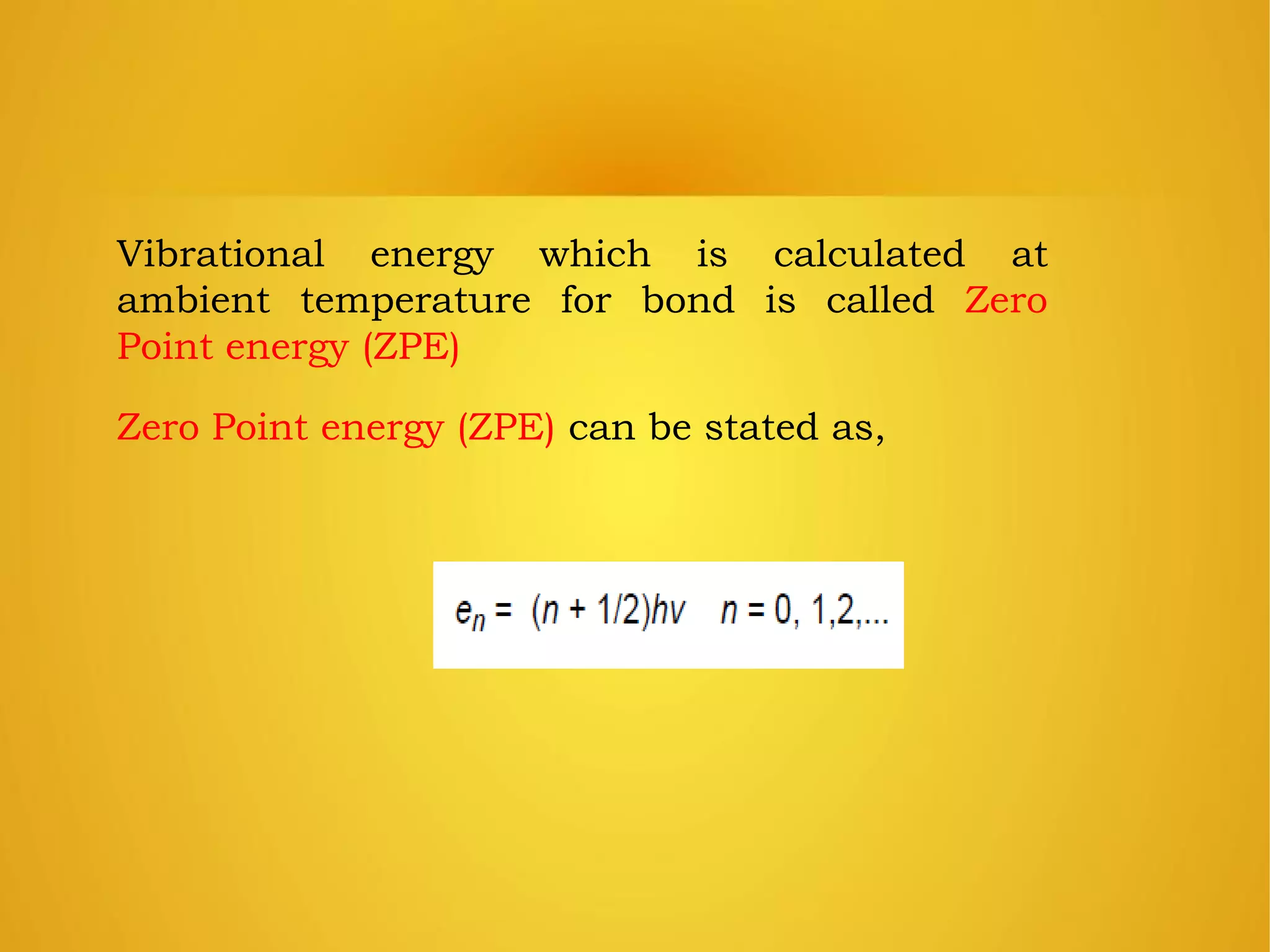
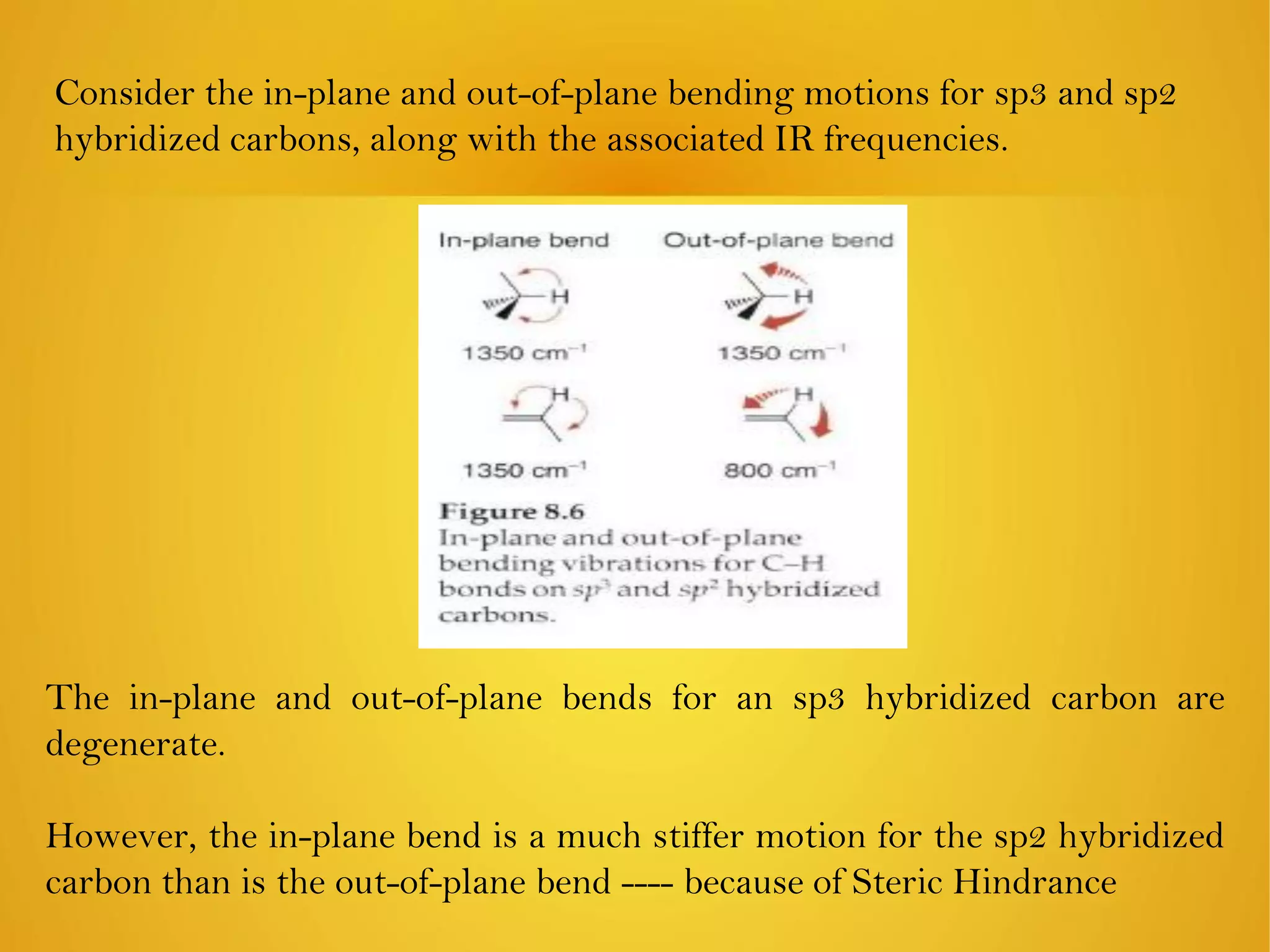
An element is identified by its symbol, atomic number, and mass number. Isotopes are atoms of the same element with the same number of protons but different numbers of neutrons. Kinetic isotope effects occur when isotopically substituted molecules react at different rates. The kinetic isotope effect is expressed as a ratio of rate constants for reactions involving different isotopes. Primary kinetic isotope effects occur when a bond to the isotopically labeled atom is broken or formed. Secondary kinetic isotope effects occur when rehybridization is involved or the isotope is remote from the changing bond. Differences in vibrational frequencies between bonds lead to different zero-point energies and measurable kinetic isotope effects.