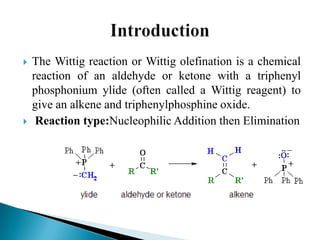
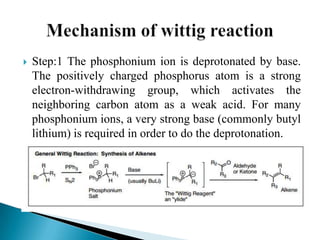
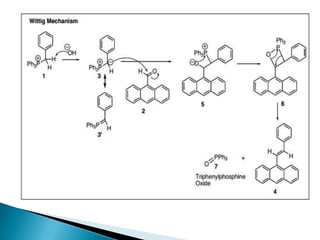
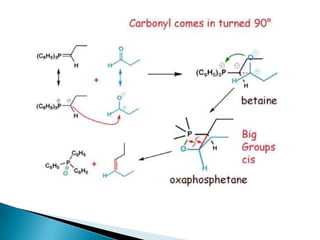
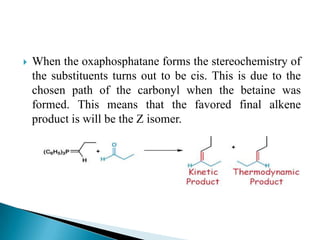
The Wittig reaction involves reacting an aldehyde or ketone with a triphenylphosphonium ylide to form an alkene and triphenylphosphine oxide. The reaction proceeds through betaine and oxaphosphetane intermediates. The stereochemistry of the product can be controlled by the reactants and conditions. Similar reactions are the Horner and Horner-Emmons-Wadsworth reactions, which use different reagents. The Wittig reaction is one of the main methods for synthesizing alkenes from carbonyl compounds.