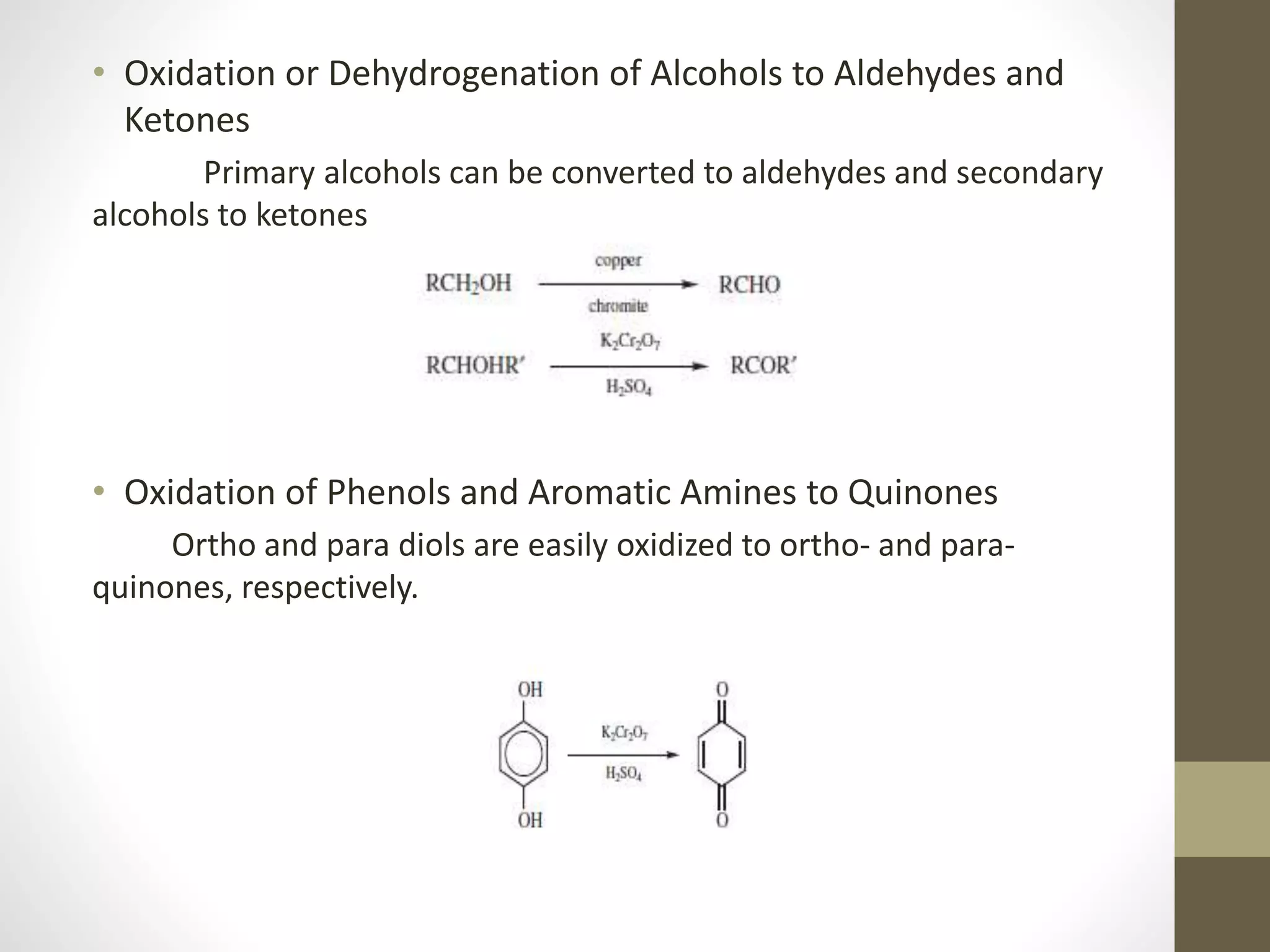
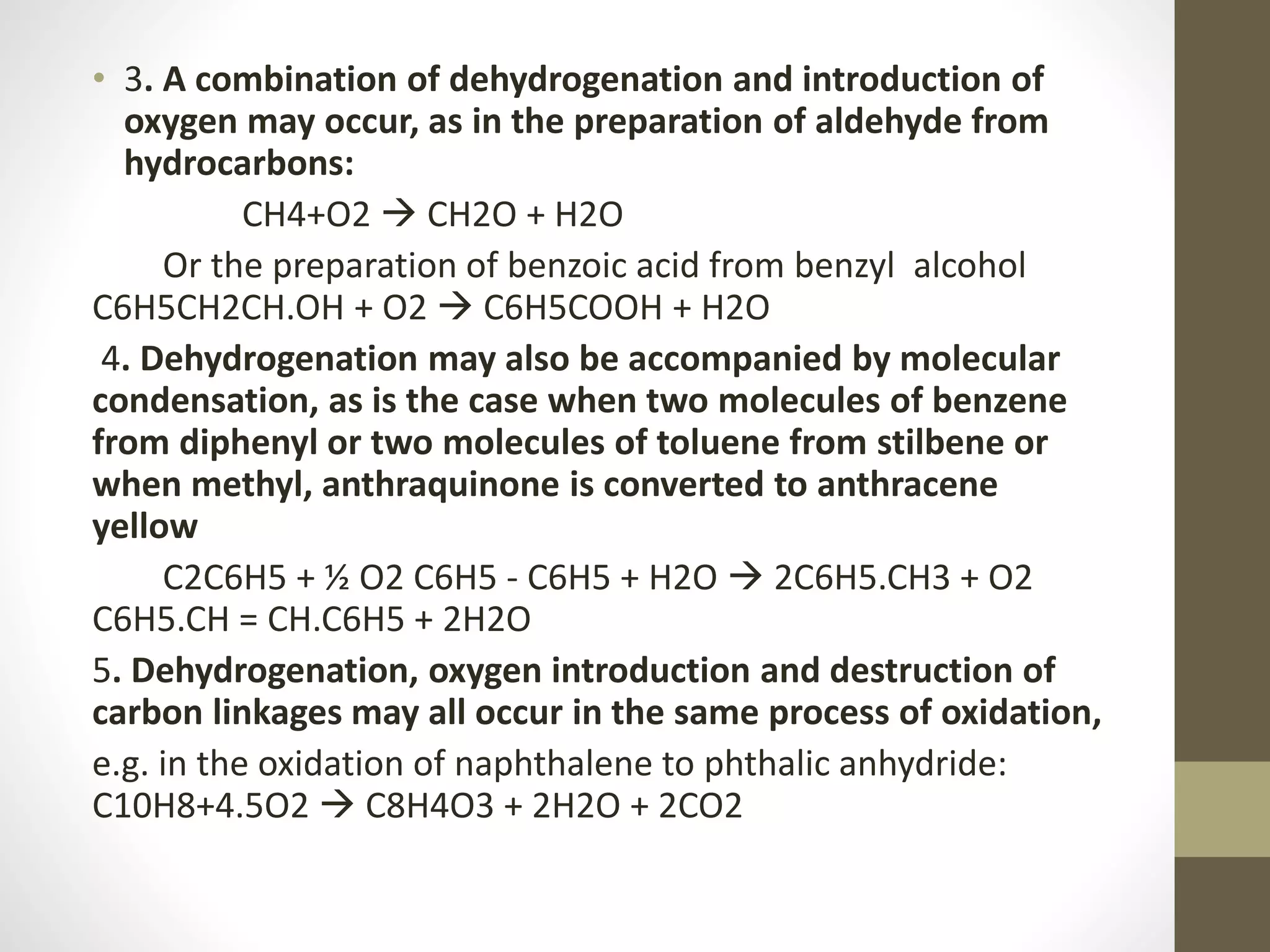
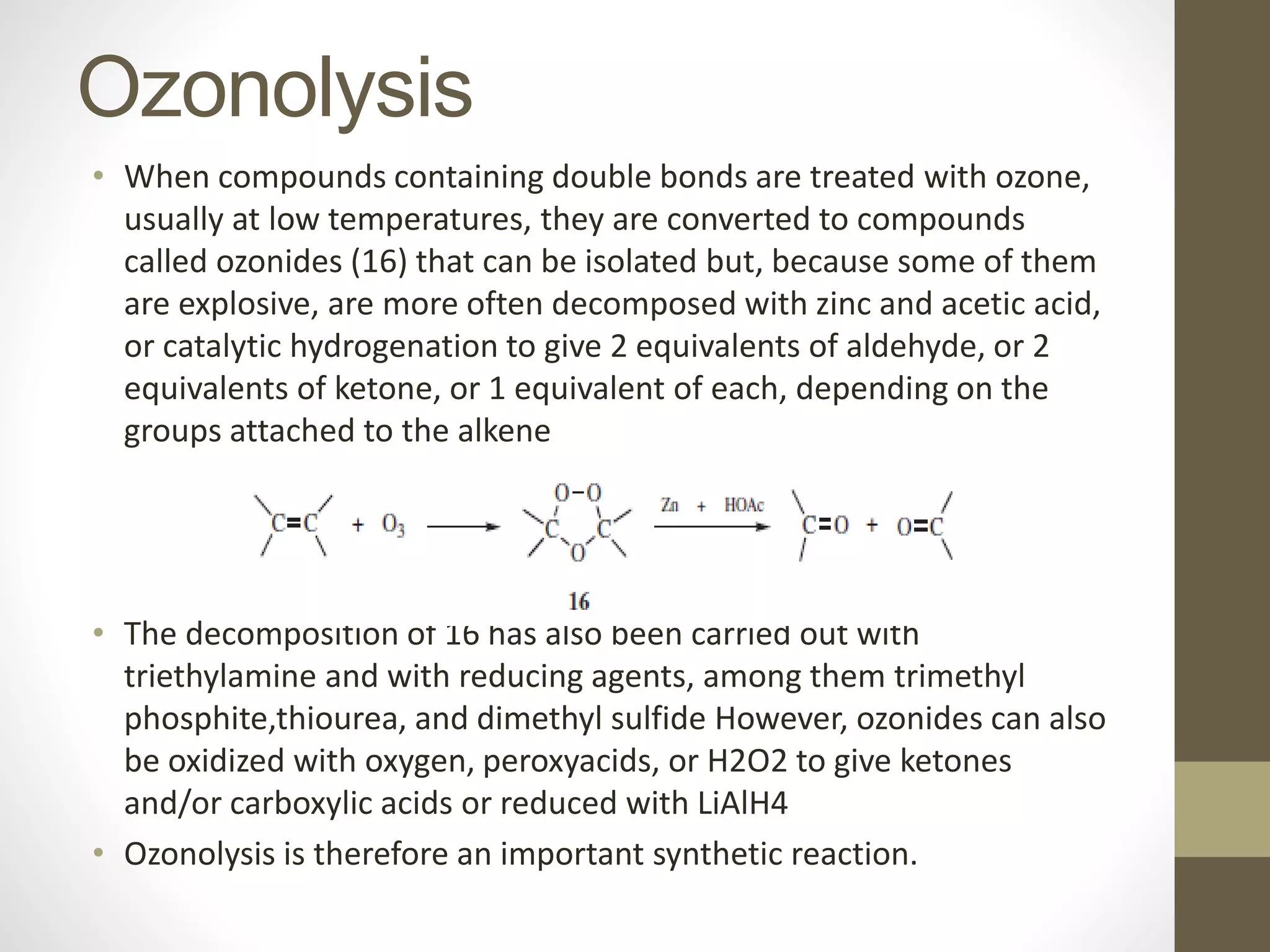
1. Oxidation is any chemical reaction that involves the transfer of electrons from one substance to another. Iron rusting is a common example of oxidation where iron reacts with oxygen and loses electrons.
2. There are several types of oxidative reactions including dehydrogenation, introduction of oxygen into a molecule, and combinations of dehydrogenation and oxygen introduction.
3. Liquid phase oxidation involves free radical chain reactions and is used to convert petroleum-based materials into commodity chemicals. Hydroperoxide is often a major product.