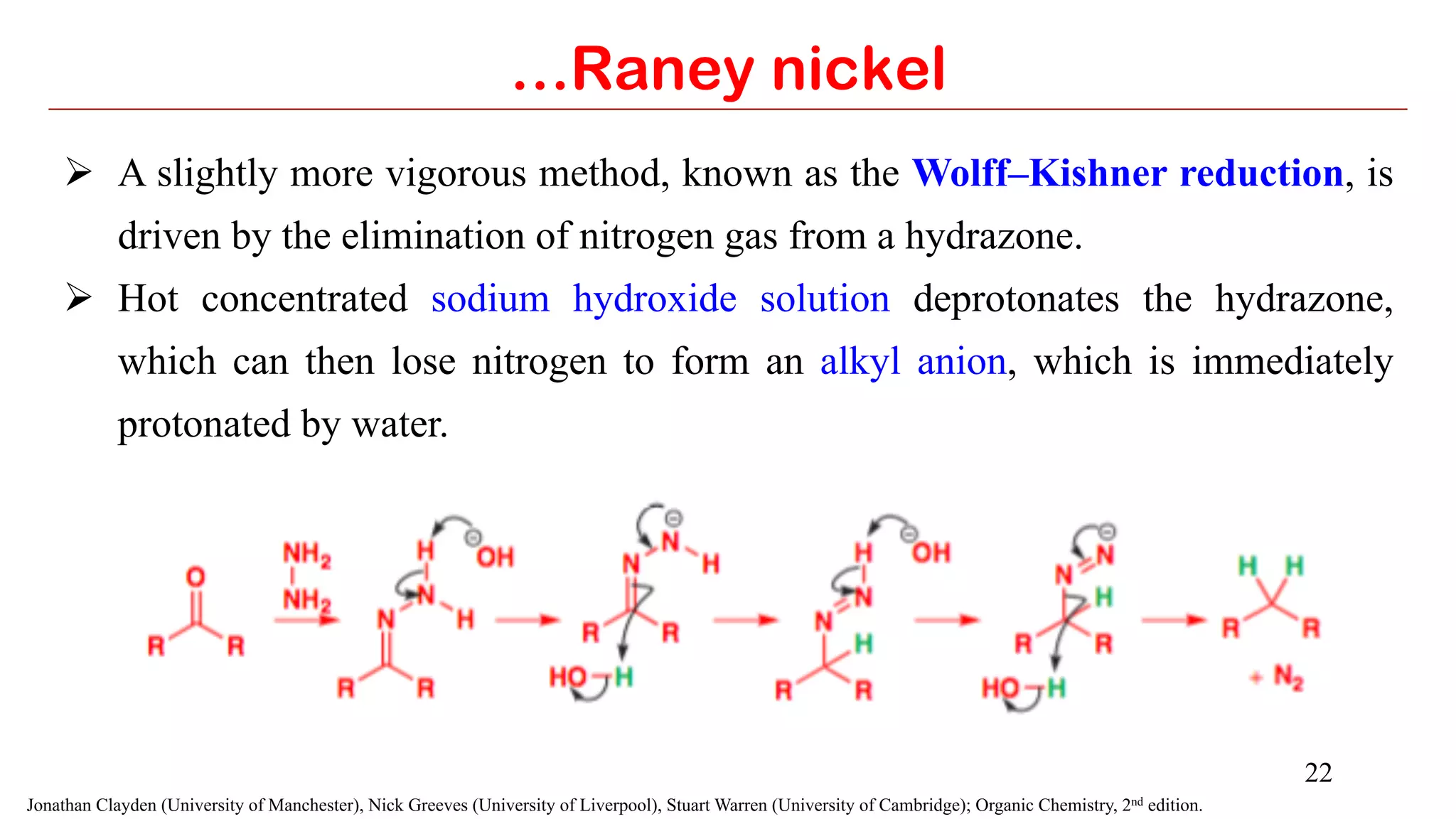
Selenium dioxide (SeO2) and Raney nickel are both useful reagents in organic synthesis. SeO2 can be used to oxidize alkenes to allylic alcohols or carbonyls. It also oxidizes carbonyls to 1,2-dicarbonyls and internal alkynes to 1,2-dicarbonyls. Raney nickel catalyzes hydrogenation of aromatics and reduction of carbonyl groups by cleaving C-S bonds. Both reagents have applications in functional group transformations.




![6
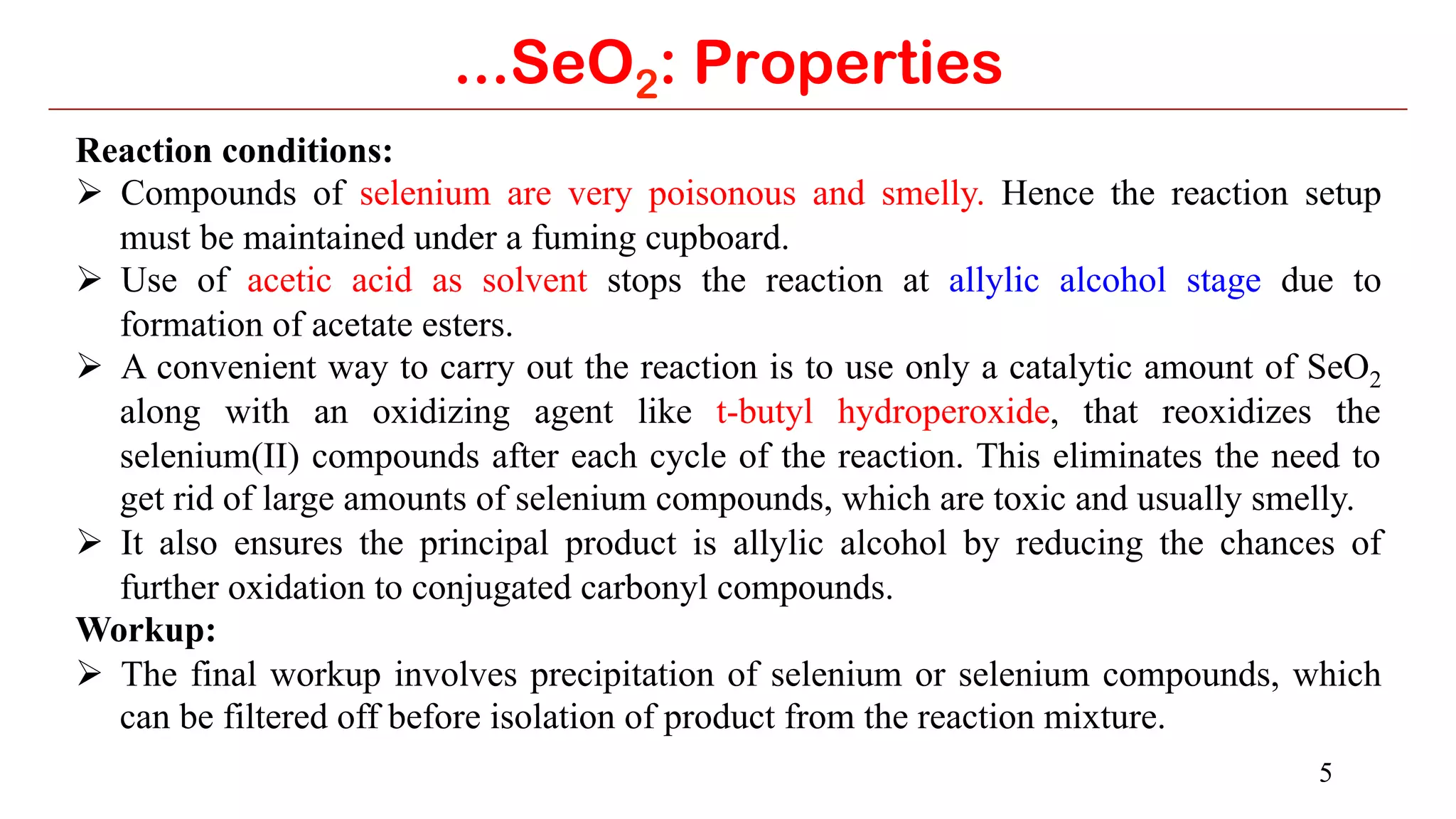
...SeO2: Applications
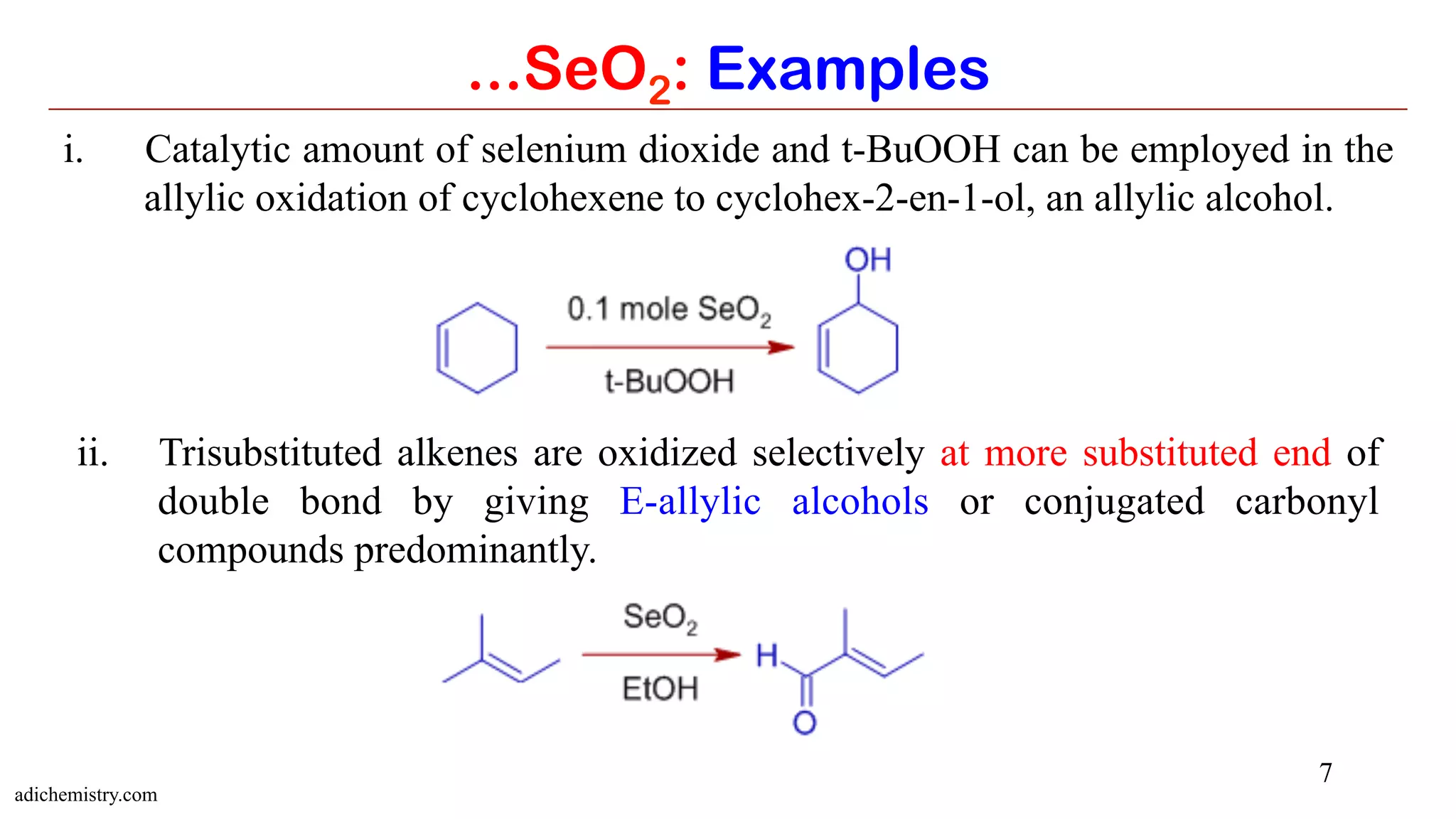
1. Allylic oxidation of alkenes:
Selenium dioxide oxidizes allylic positions to alcohol or carbonyl groups. It starts with
Alder-ene like 4+2 cycloaddition of SeO2 to give an allylic selenic acid that further
undergoes [2,3]-sigmatropic rearrangement to give an unstable compound that may
decompose to allylic alcohol or an allylic carbonyl compound as shown below.
adichemistry.com](https://image.slidesharecdn.com/13-210514083413/75/13-SeO2-amp-raney-ni-6-2048.jpg)
![9
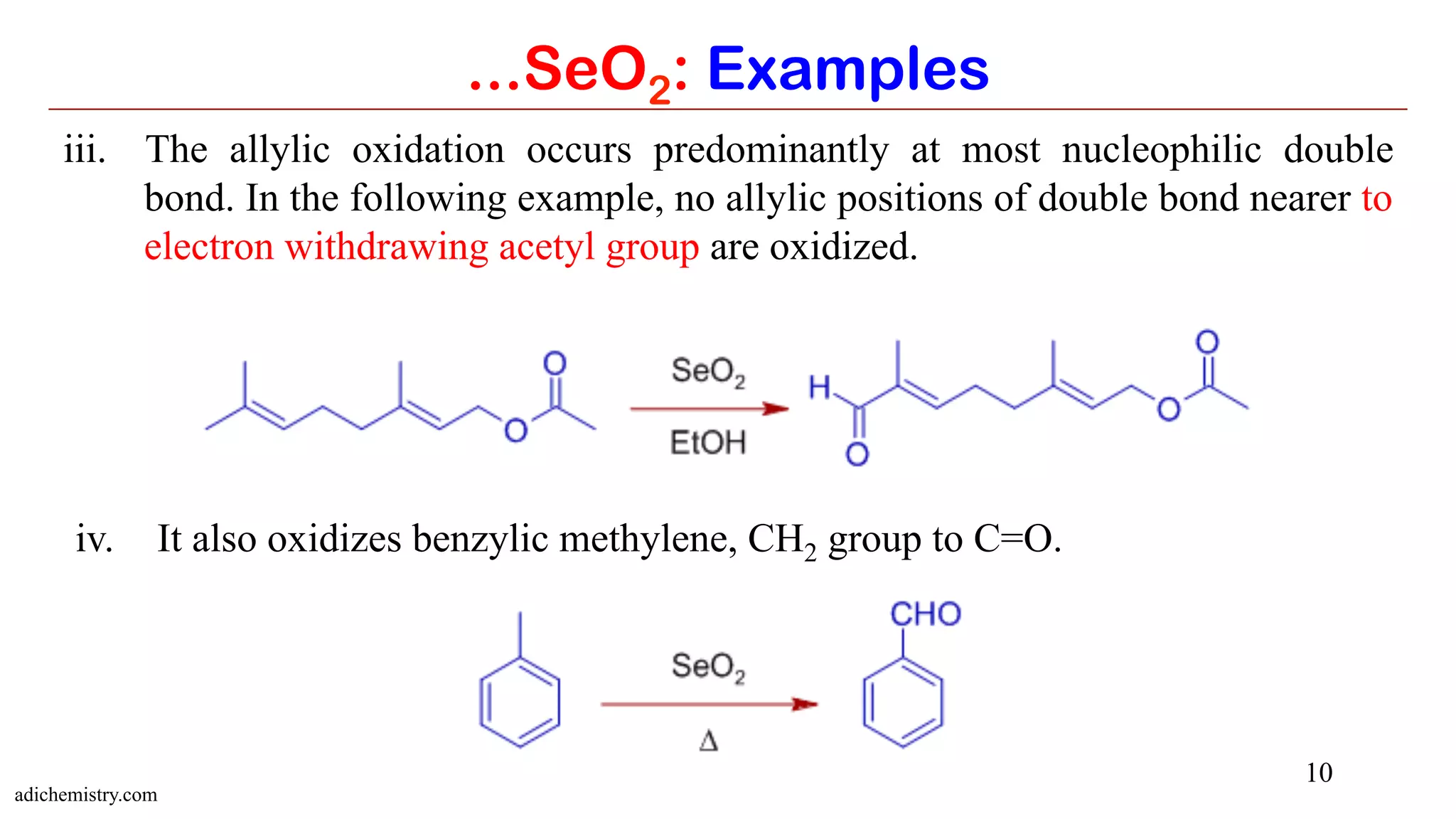
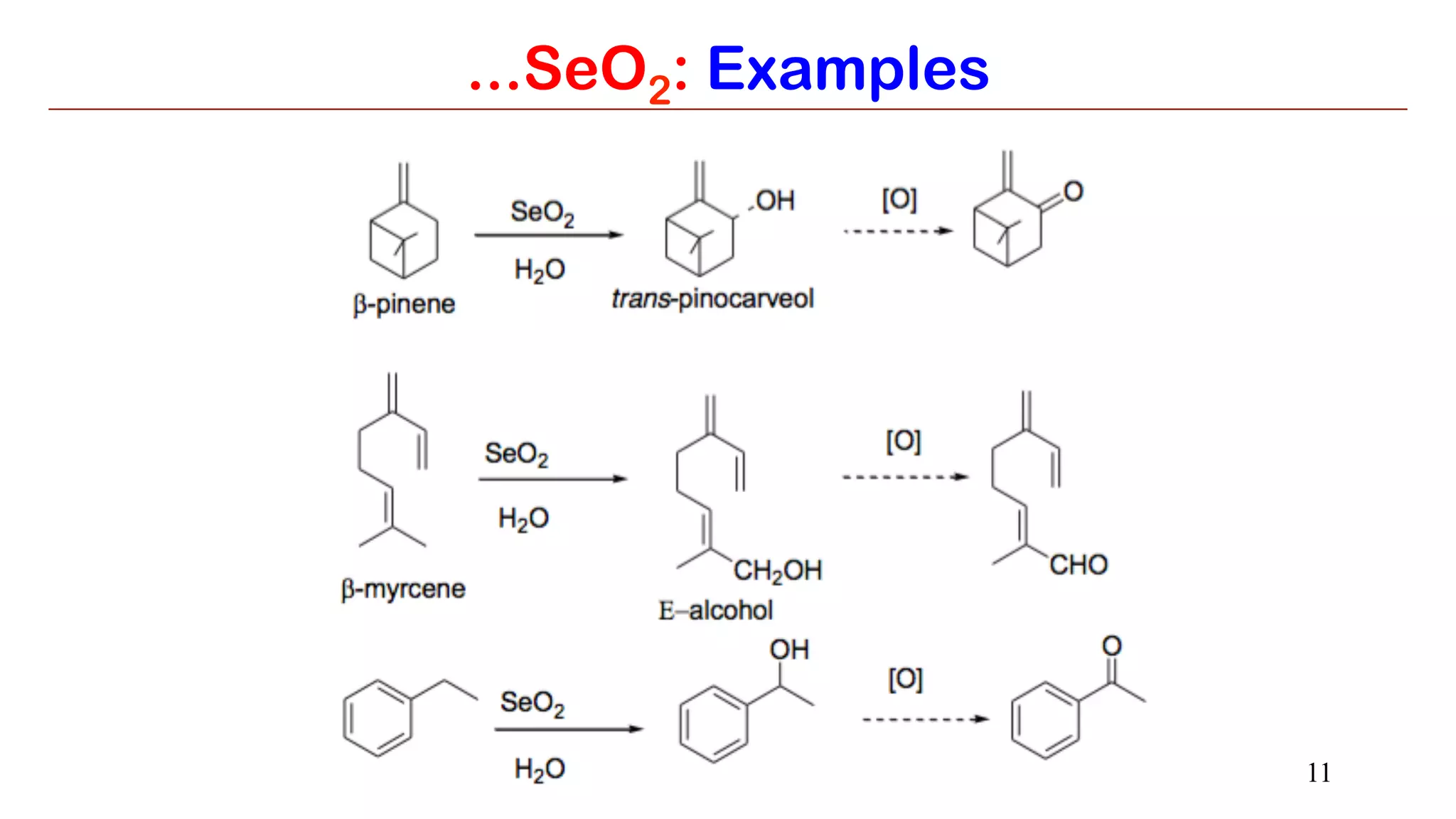
...SeO2: Examples
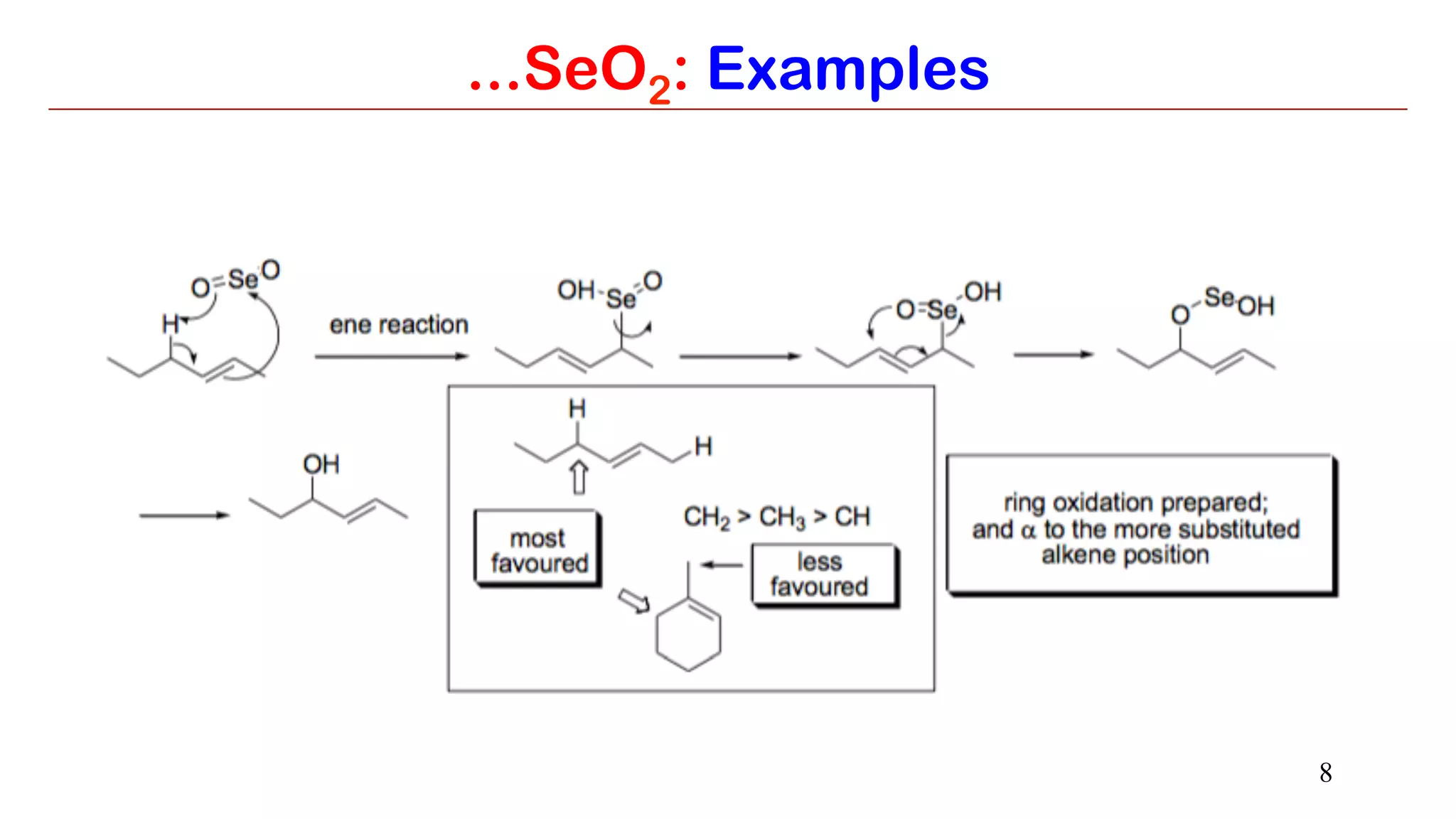
It is because the initial ene type 4+2 cycloaddition involves preferential attack of the
more nucleophilic end of double bond at selenium. In this step, alkene uses the π-
HOMO to attack the π*-LUMO of Se=O. Meanwhile the π-HOMO of Se=O attacks the
σ*-LUMO of C-H of the allylic system.
The E-selectivity is due to cyclic nature of final [2,3] sigmatropic step in which
the alkyl substituent adopts pseudoequatorial position.
adichemistry.com](https://image.slidesharecdn.com/13-210514083413/75/13-SeO2-amp-raney-ni-9-2048.jpg)