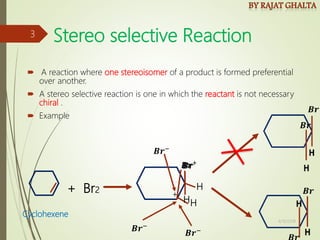
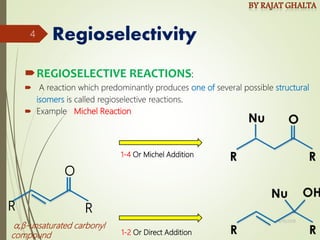
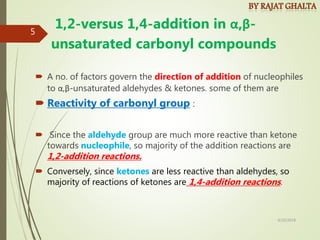
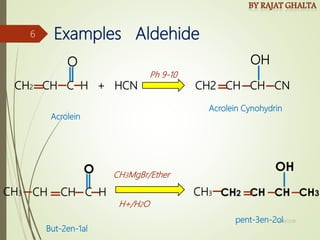
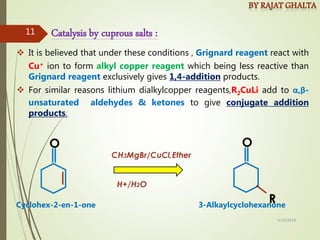
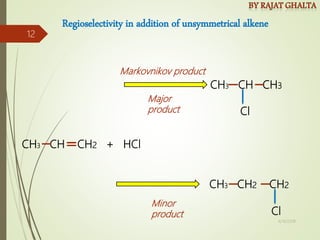
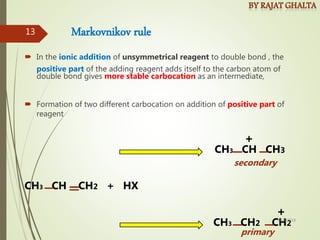
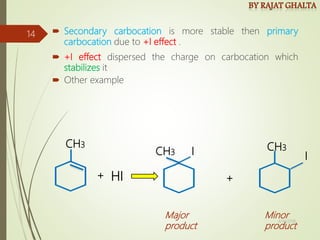
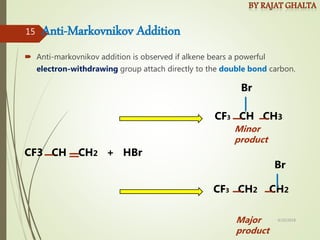
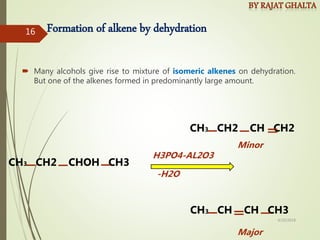
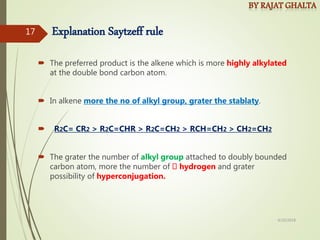
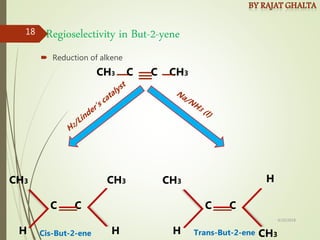
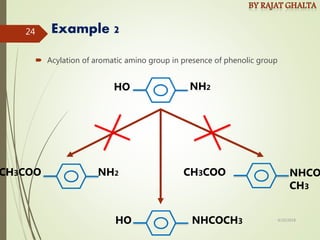
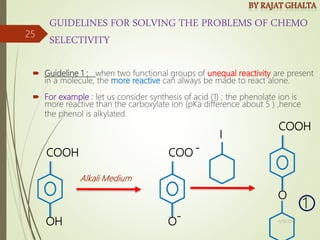
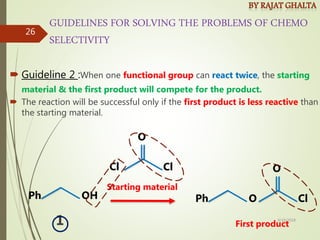
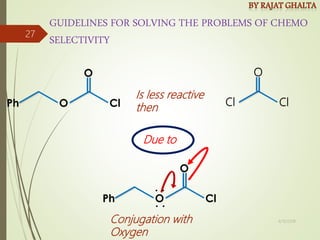
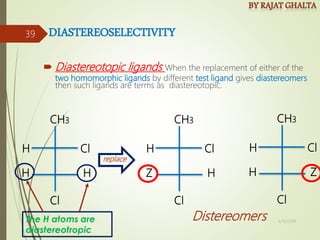
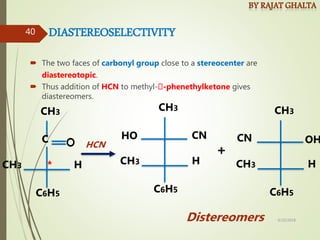
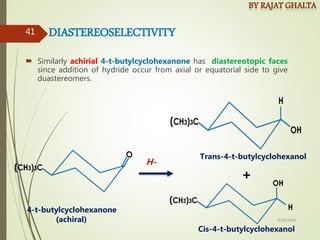
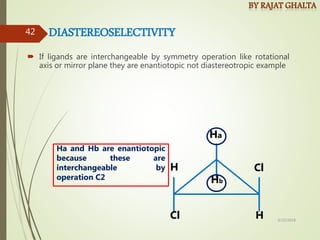
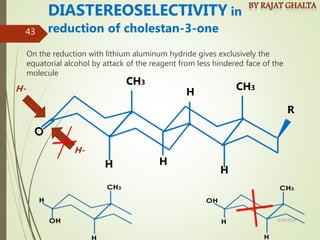
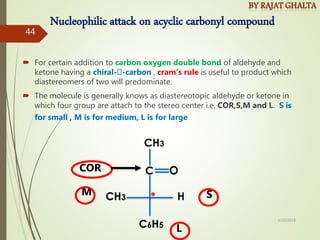
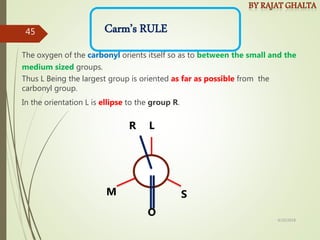
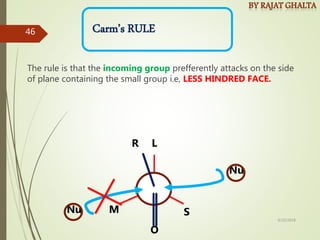
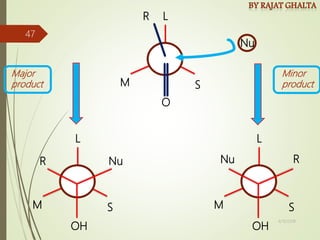
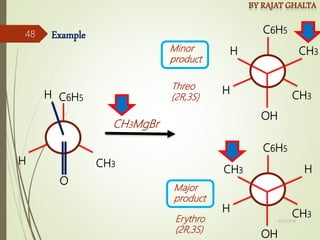
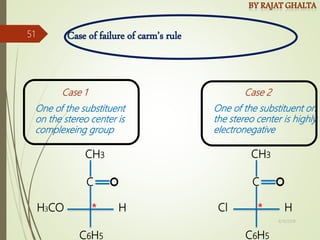
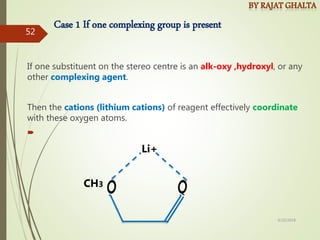
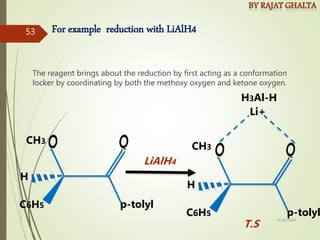
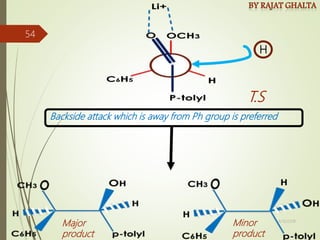
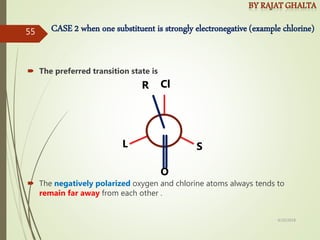
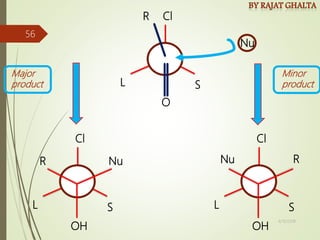
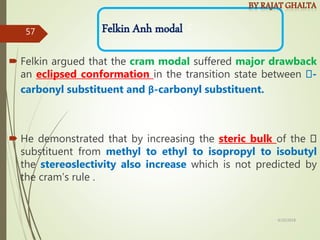
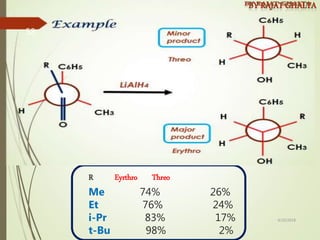
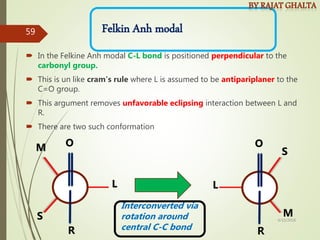
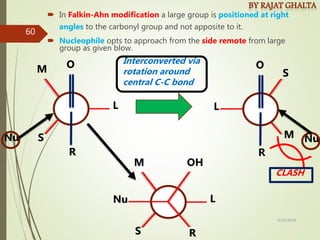
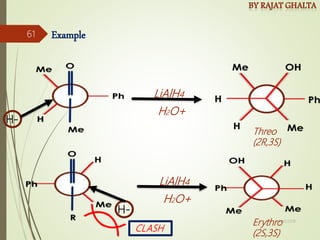
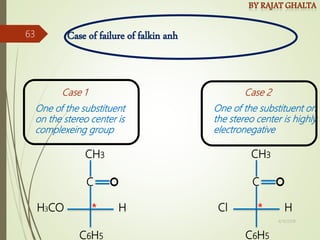
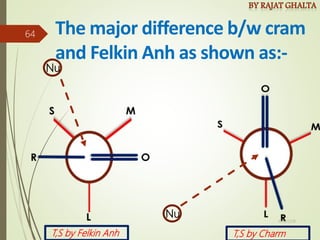
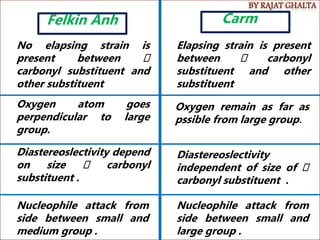
The document discusses various types of selectivity in organic reactions including:
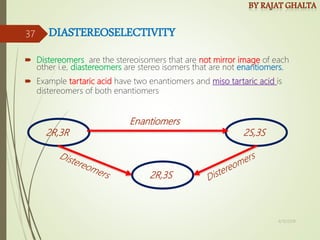
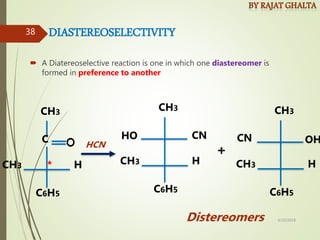
- Stereo selectivity which controls the stereochemistry of products
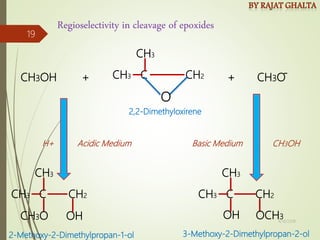
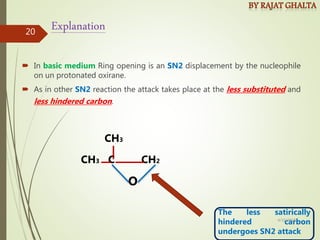
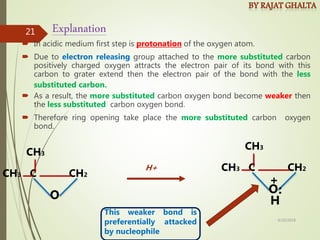
- Regioselectivity which controls the site of reaction
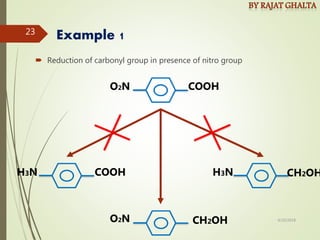
- Chemoselectivity which controls reaction of one functional group in the presence of others
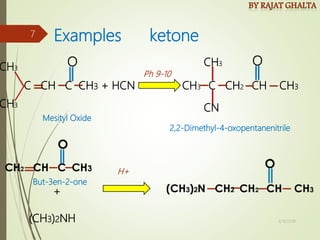
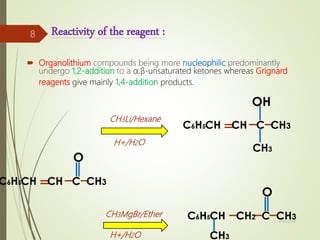
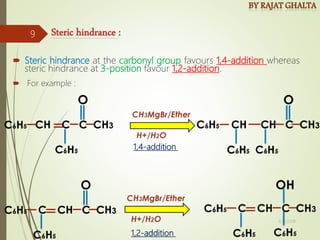
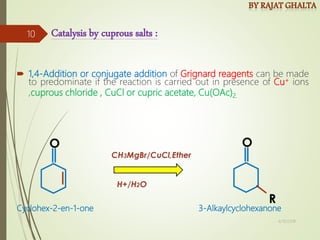
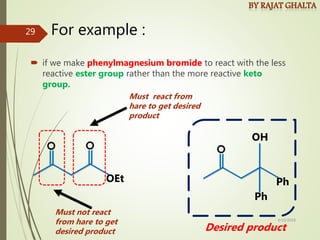
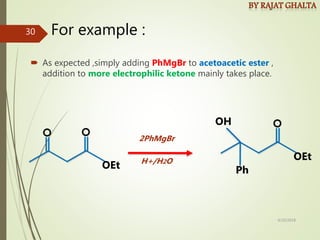
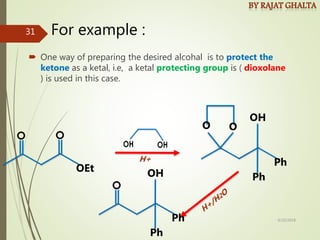
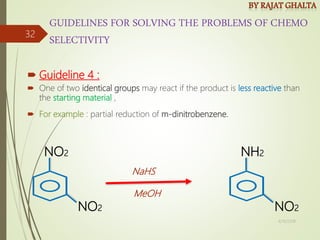
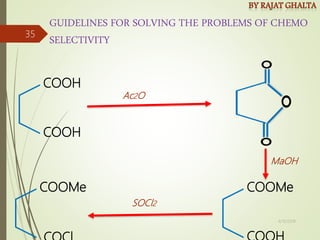
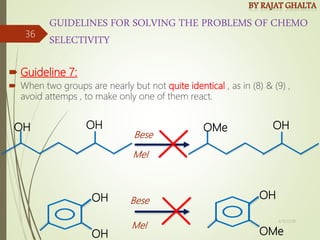
Examples and explanations are provided for each type of selectivity with mechanisms and factors that influence the outcome. Guidelines for solving problems of chemoselectivity involving protecting groups and derivatives that can react only once are also outlined.