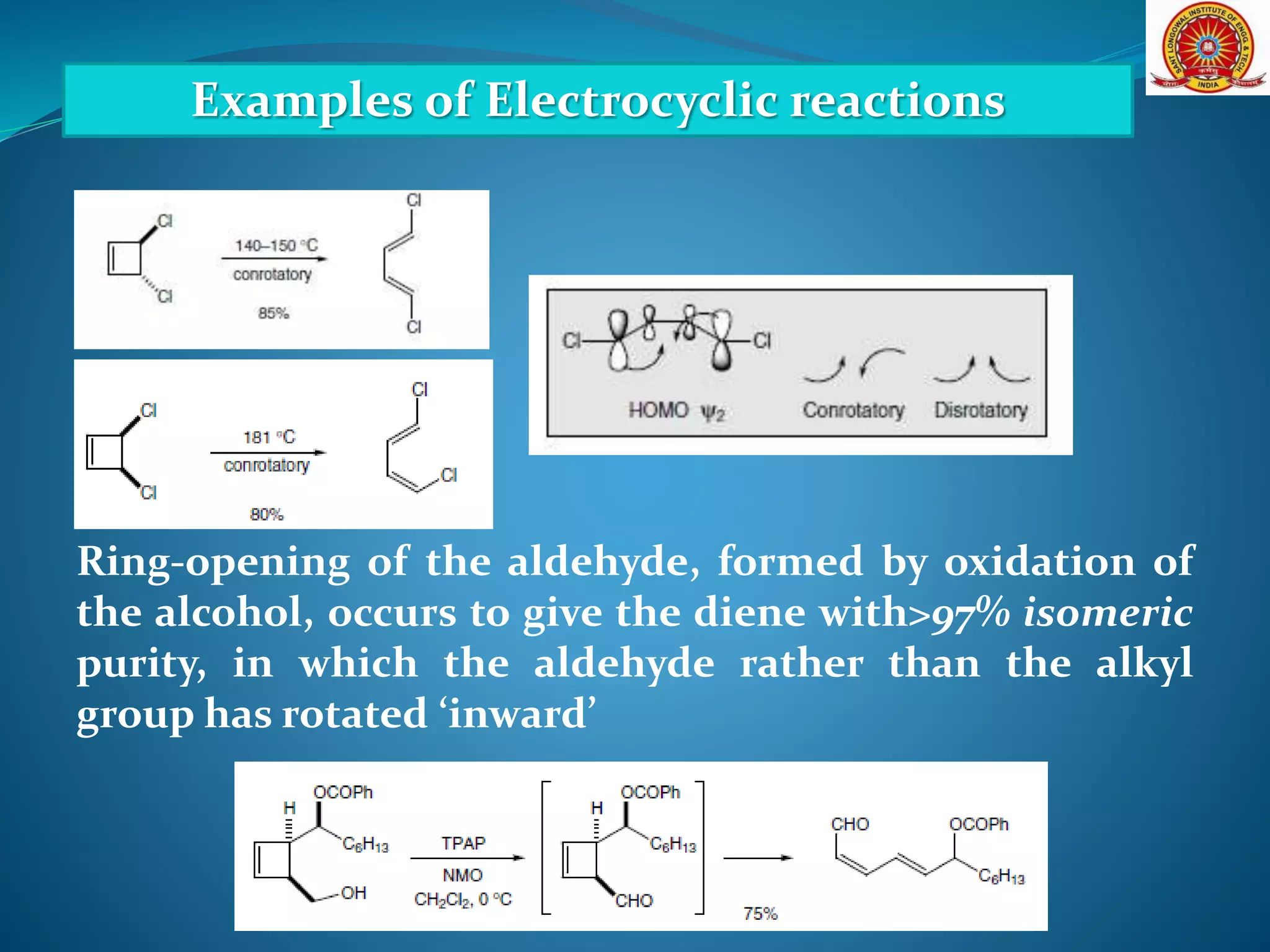
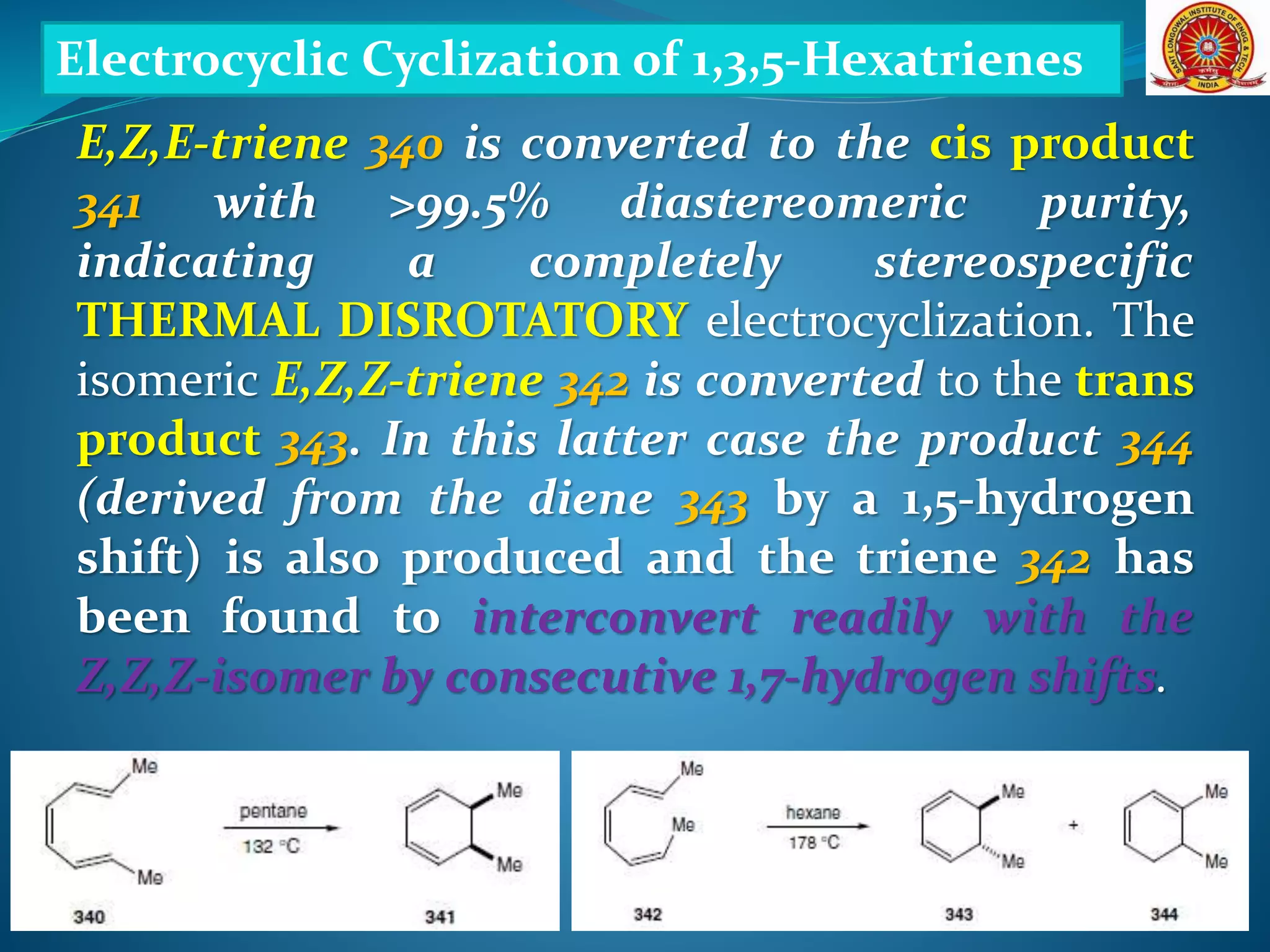
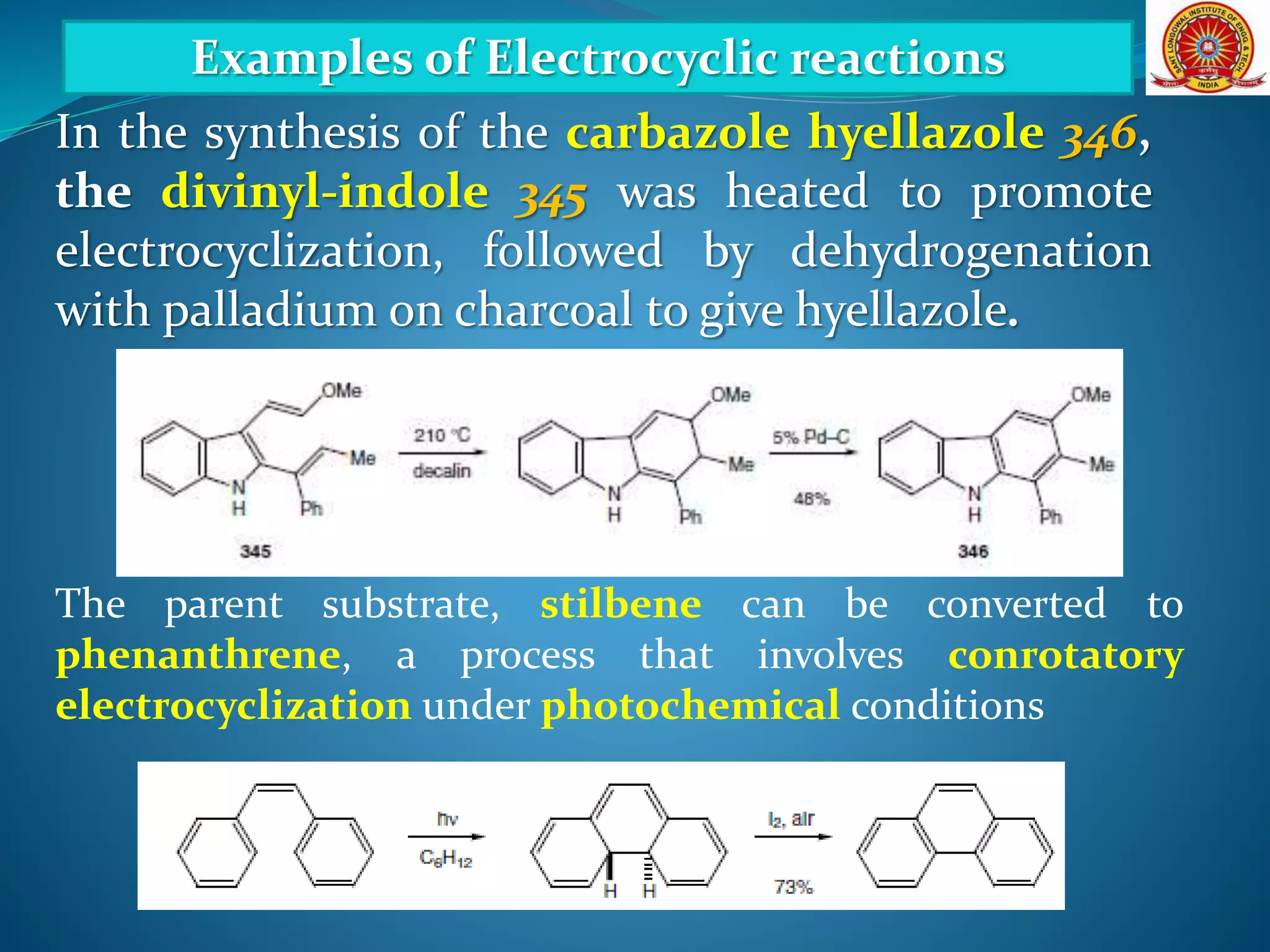
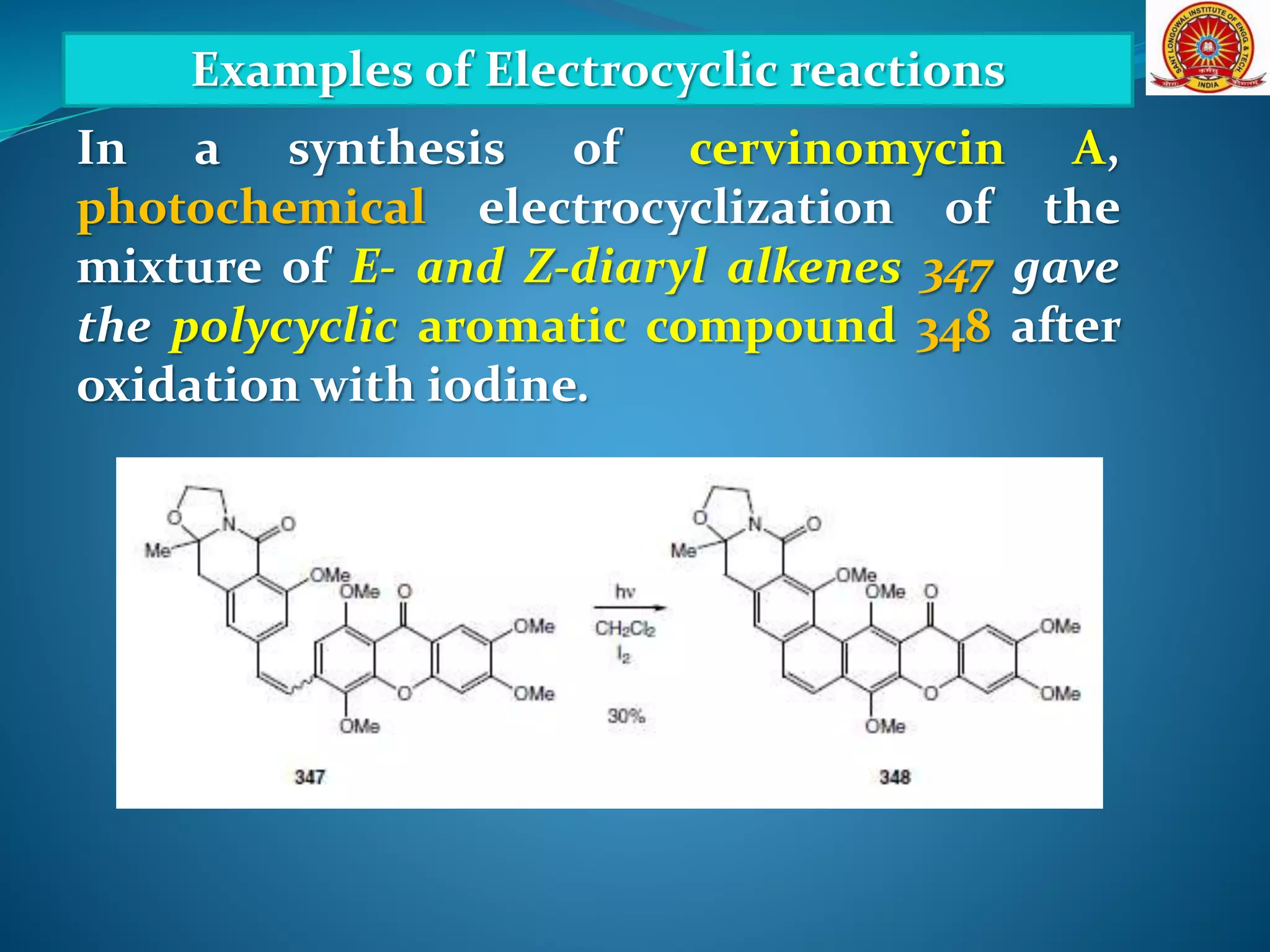
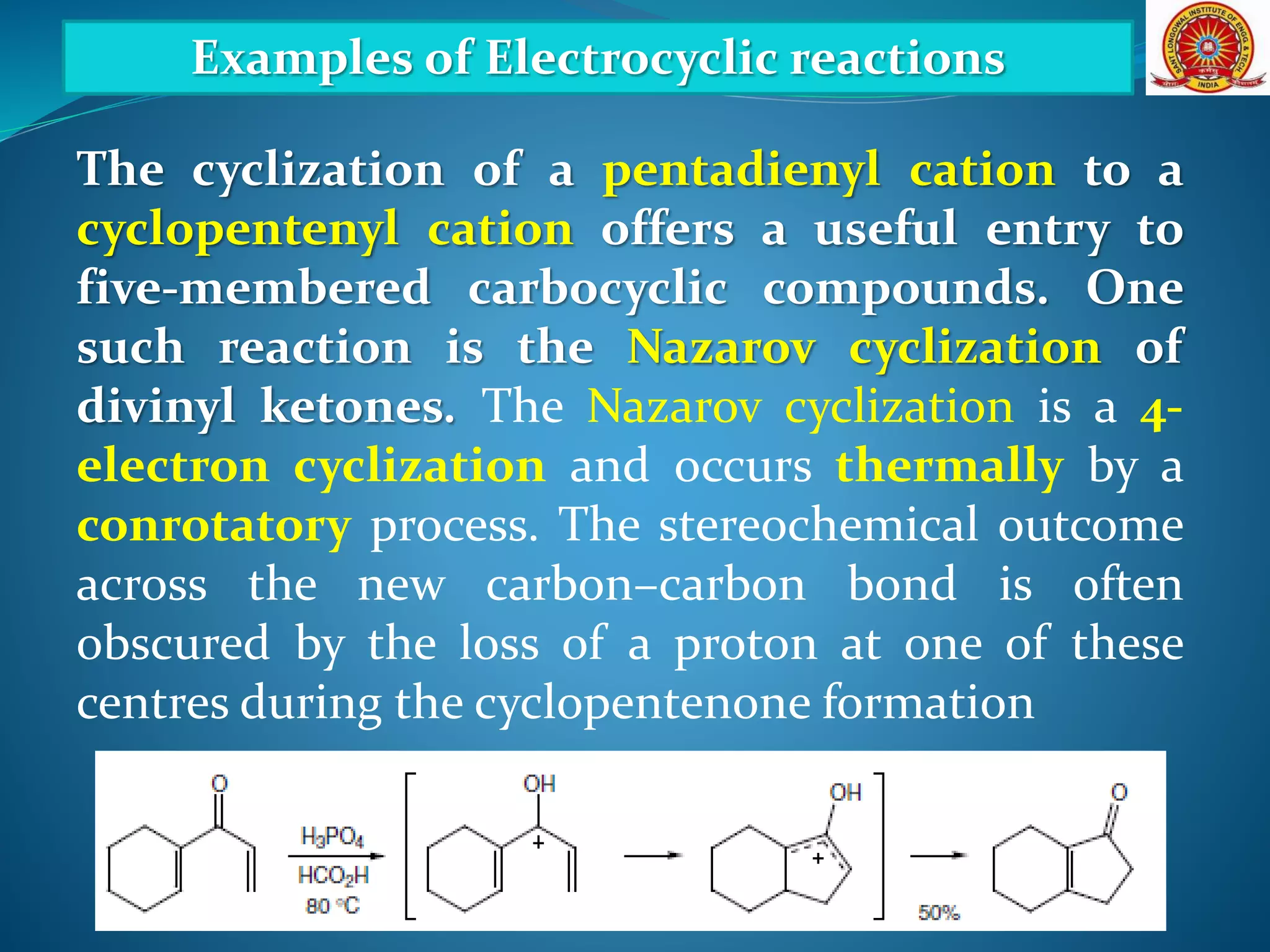
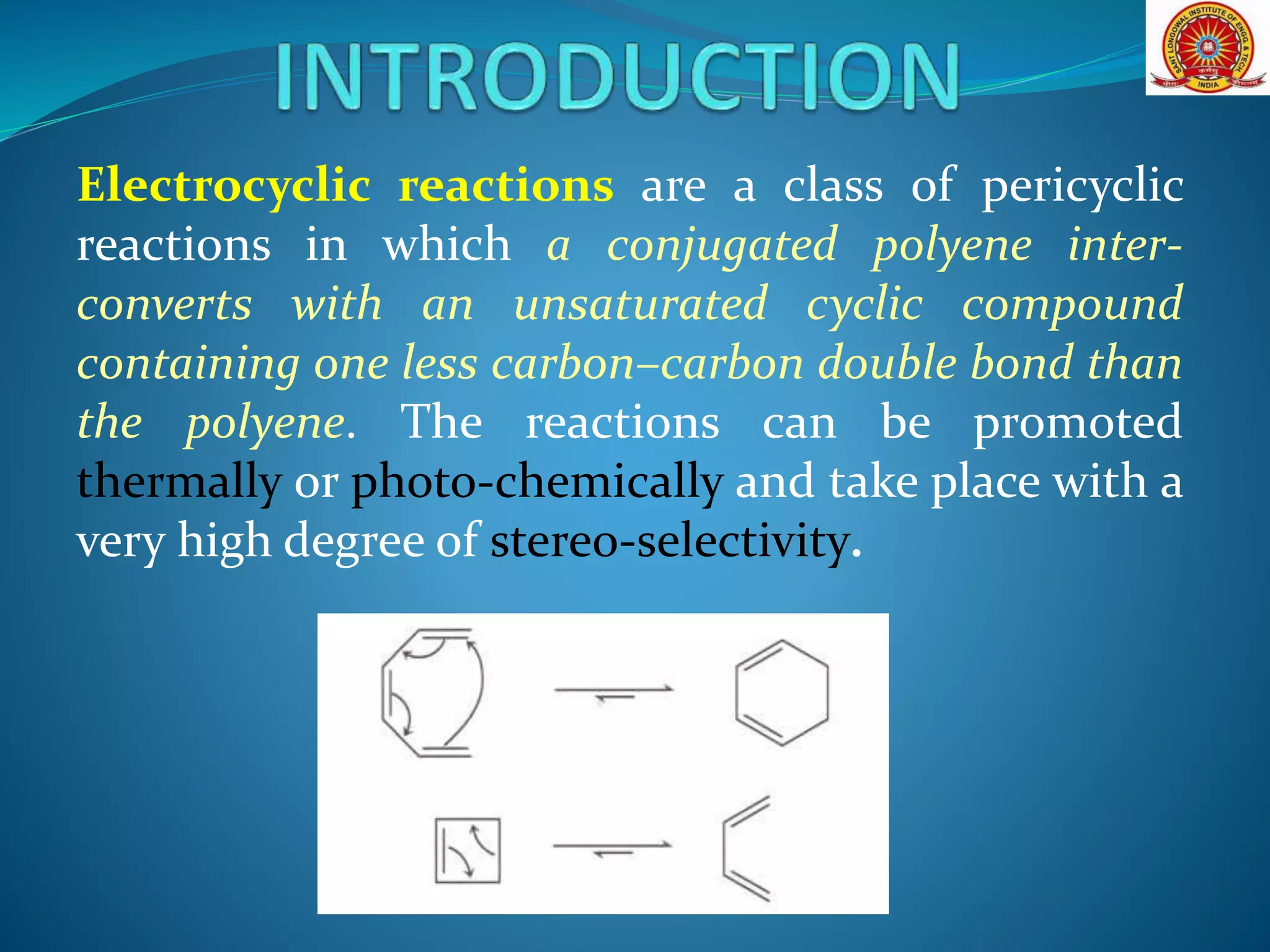
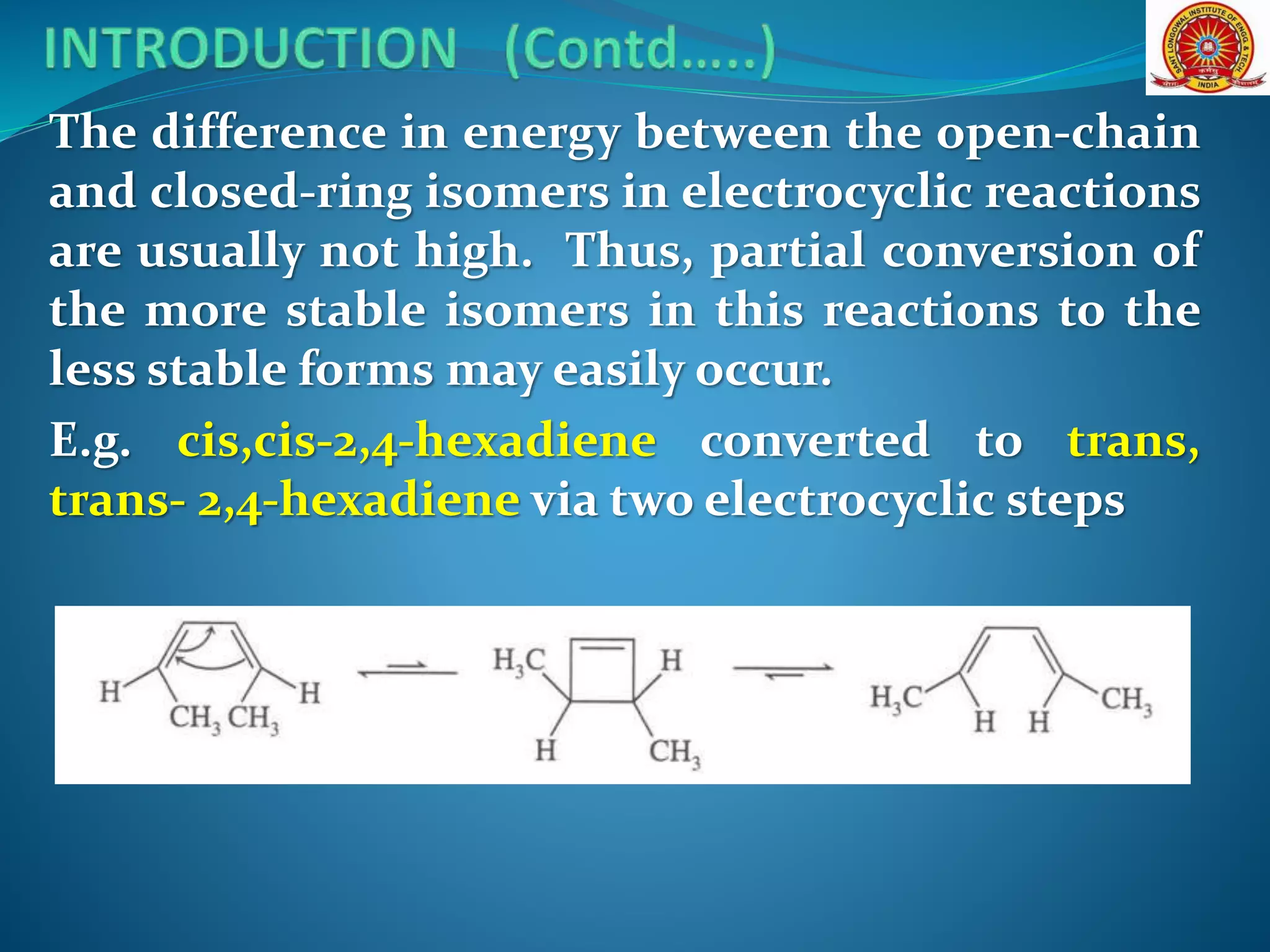
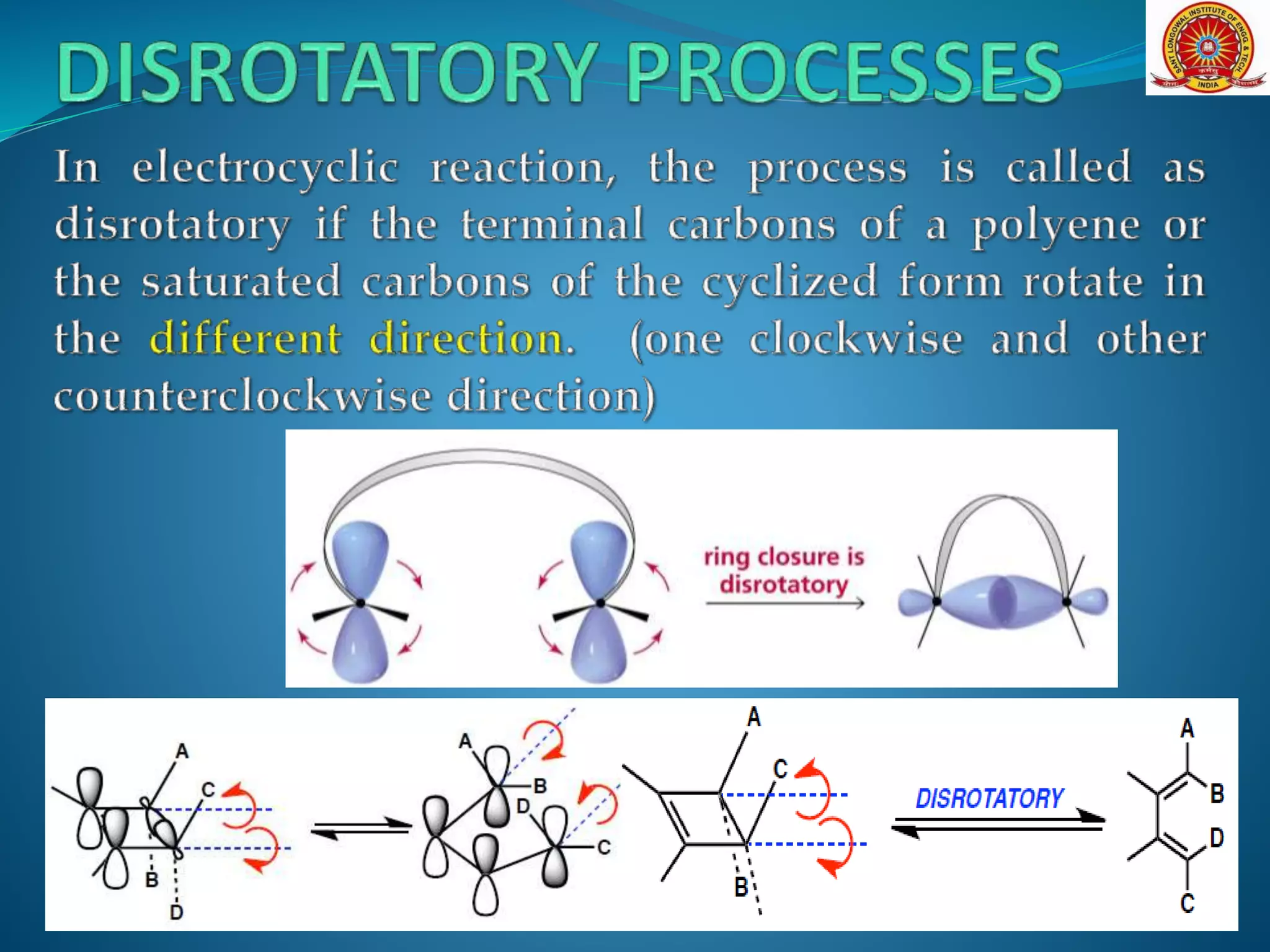
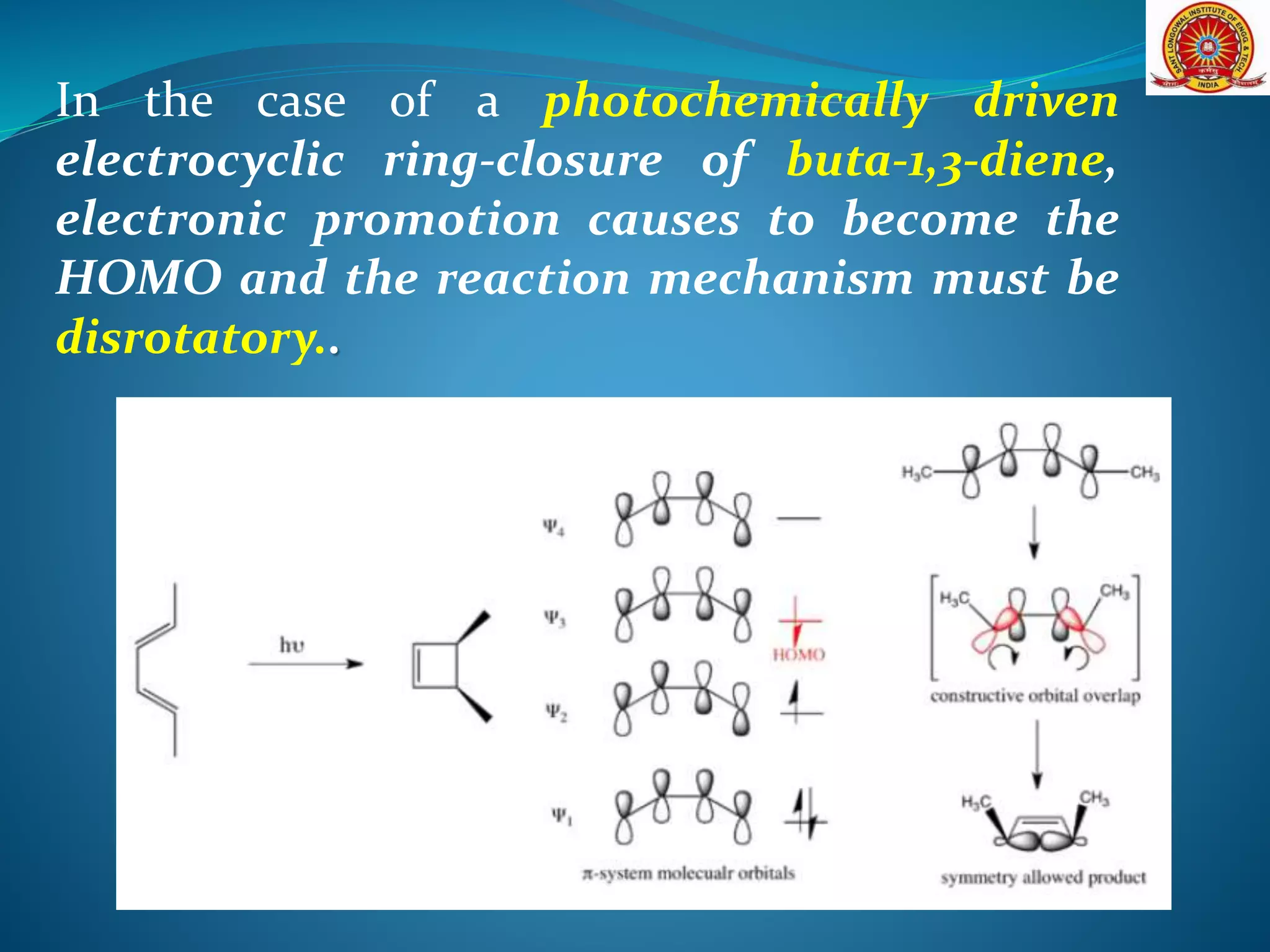
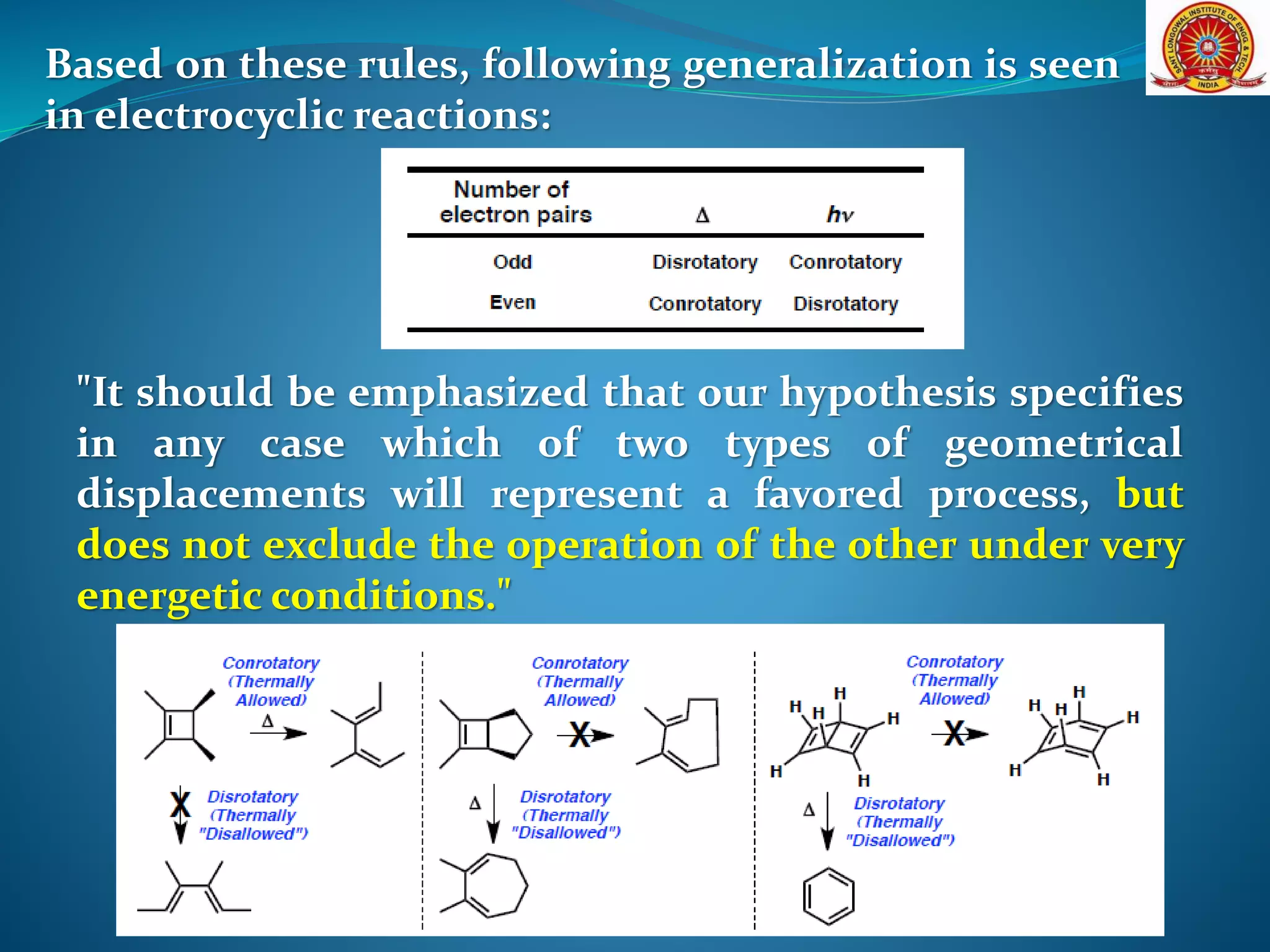
The document discusses electrocyclic reactions, which involve the conversion of a conjugated polyene to an unsaturated cyclic compound with one less carbon-carbon double bond. It notes that these reactions can occur thermally or photochemically, and with high stereoselectivity. It provides examples of electrocyclic reactions involving butadiene and hexatriene, and discusses the correlation between molecular orbital symmetry and the conrotatory or disrotatory nature of the reaction. It also addresses electrocyclic reactions involving reactants with an odd number of atoms, such as cations and anions, as well as photochemical cyclizations.









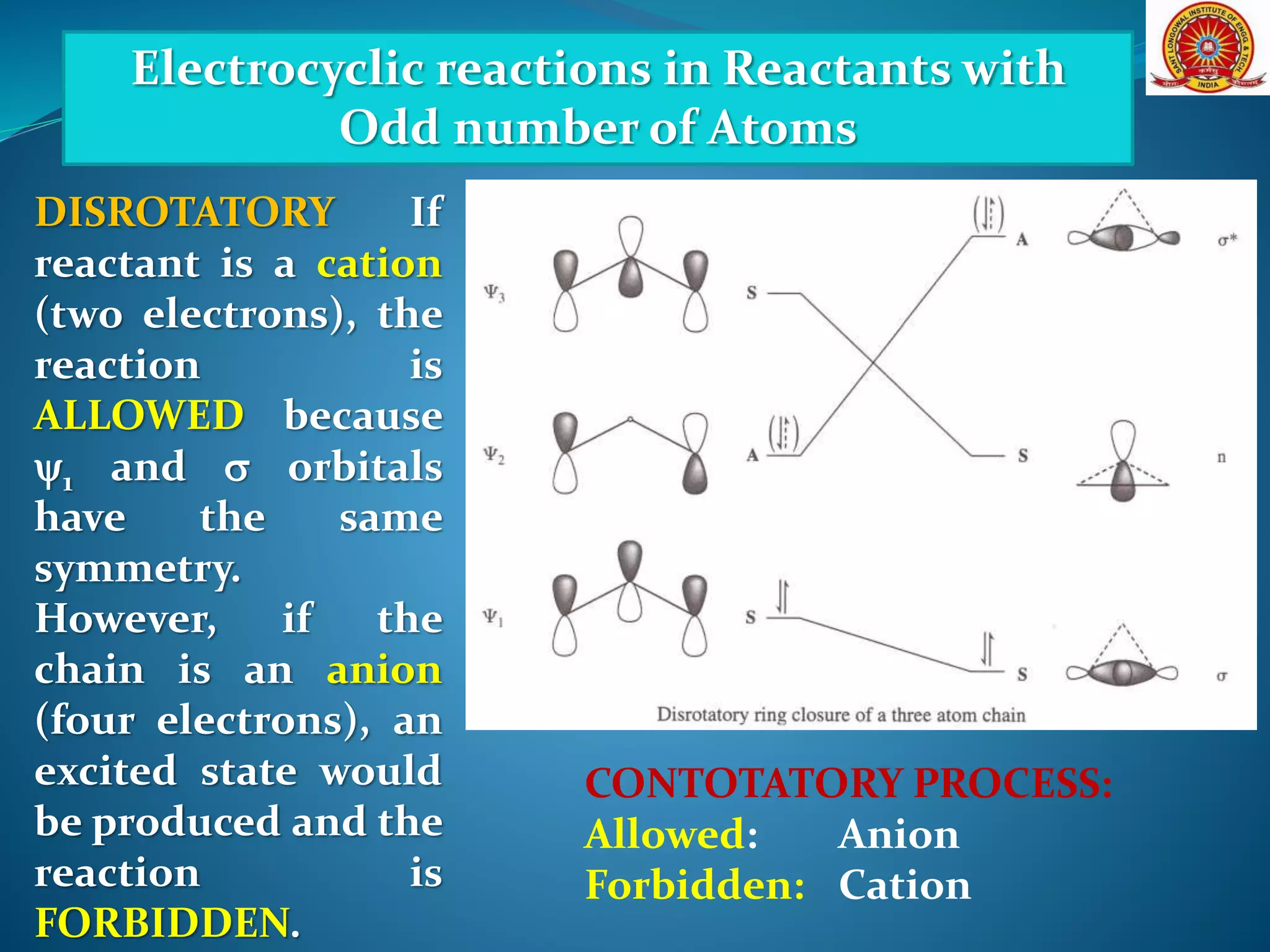

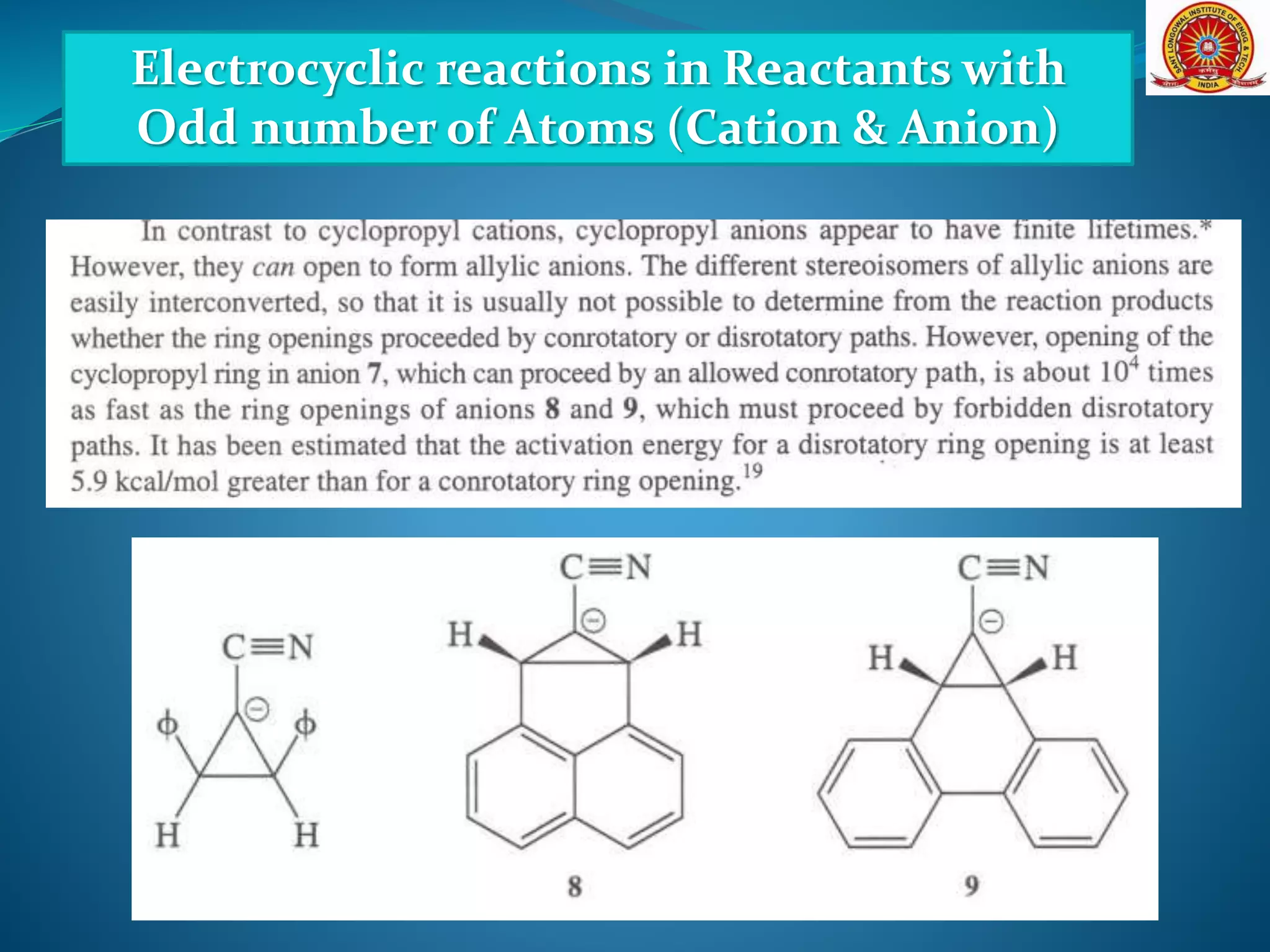
![Electrocyclic reactions in Reactants with
Odd number of Atoms (Cation & Anion)
[Formation and Cyclization of Dipolar Molecules]](https://image.slidesharecdn.com/electrocyclicreactions-200513124943/75/Electrocyclic-reactions-21-2048.jpg)
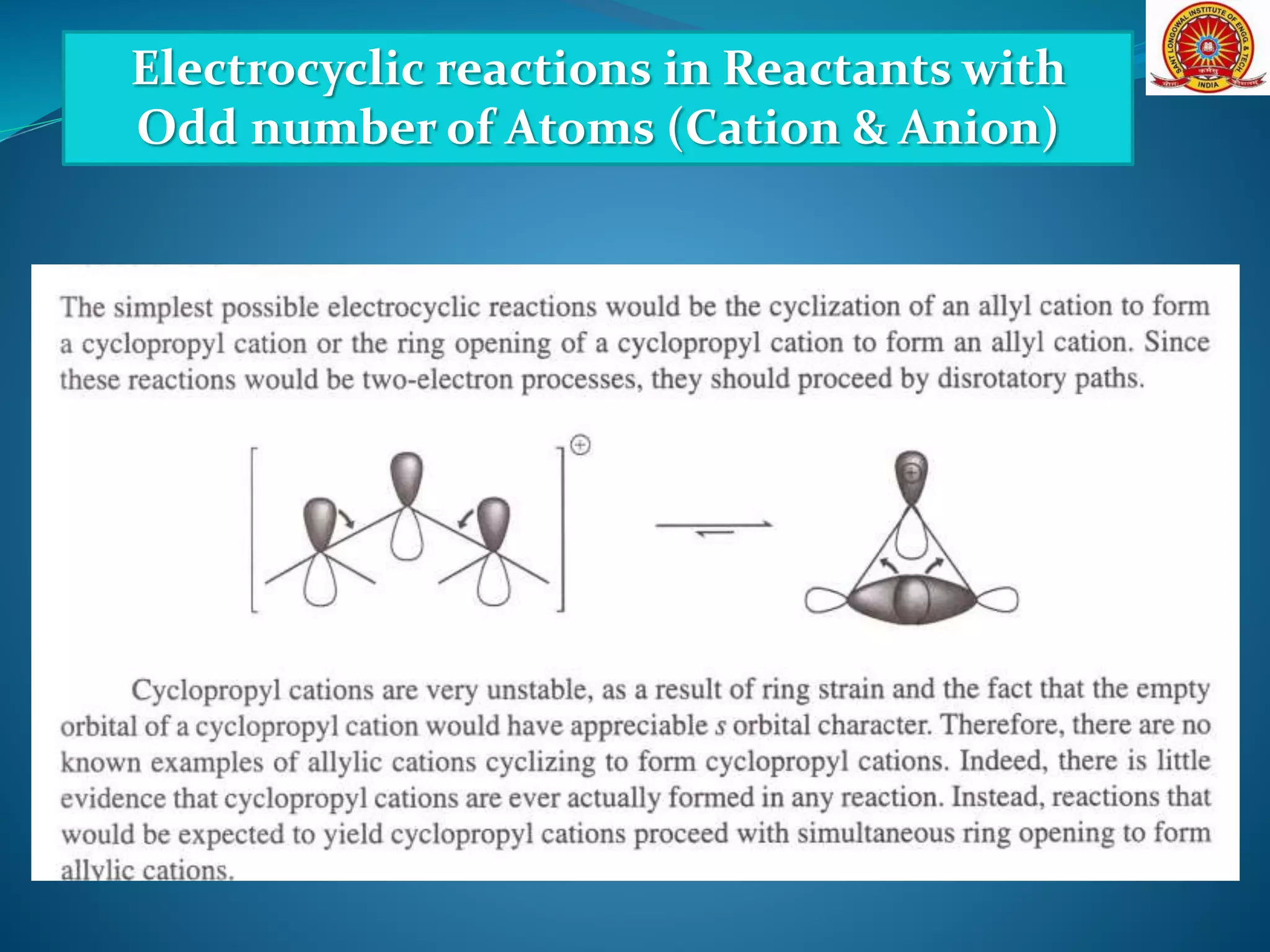
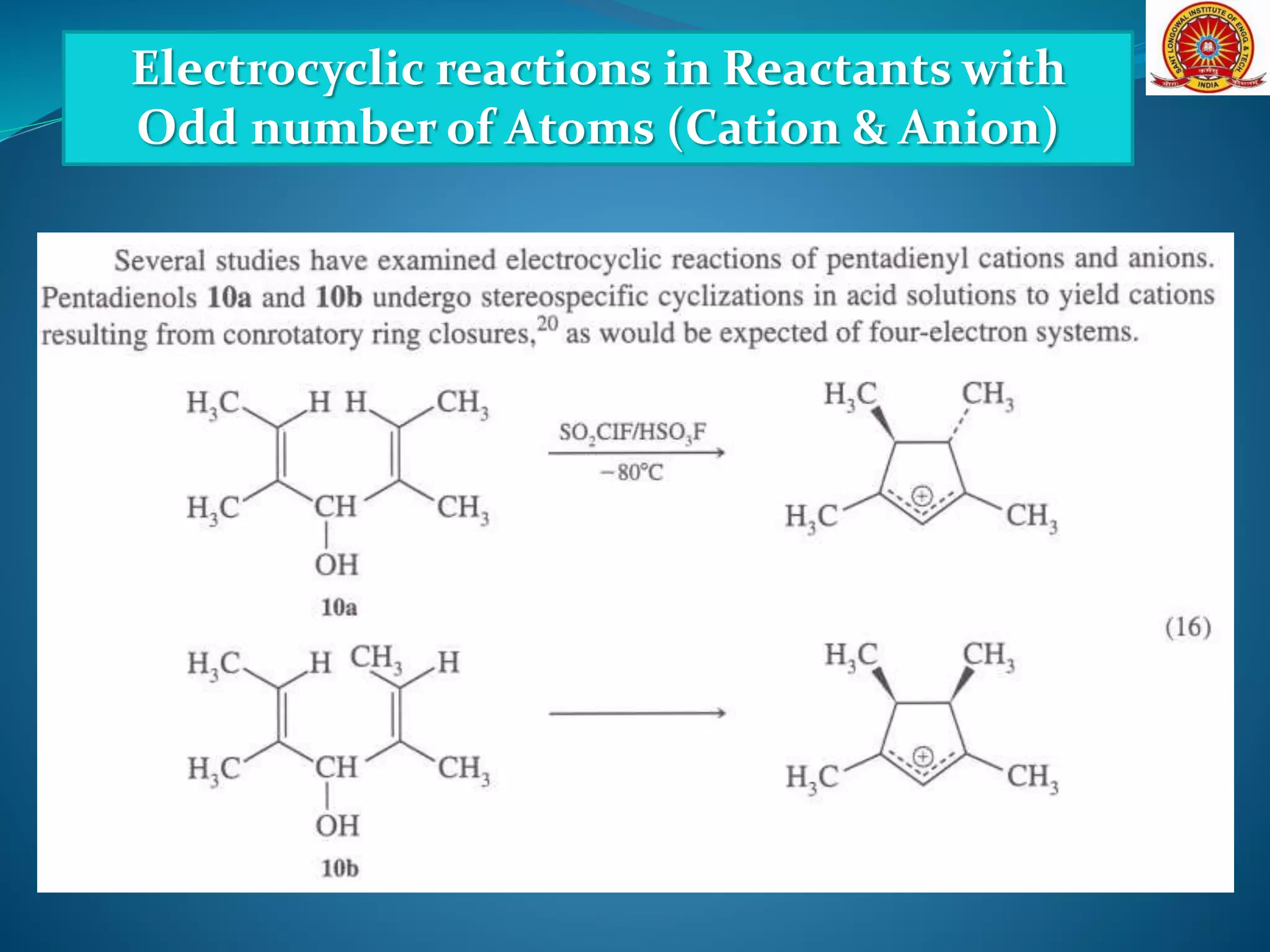
![Electrocyclic reactions in Reactants with
Odd number of Atoms (Cation & Anion)
[Formation and Cyclization of Dipolar Molecules]
The reaction
proceeds through
CONROTATORY
PROCESS](https://image.slidesharecdn.com/electrocyclicreactions-200513124943/75/Electrocyclic-reactions-22-2048.jpg)
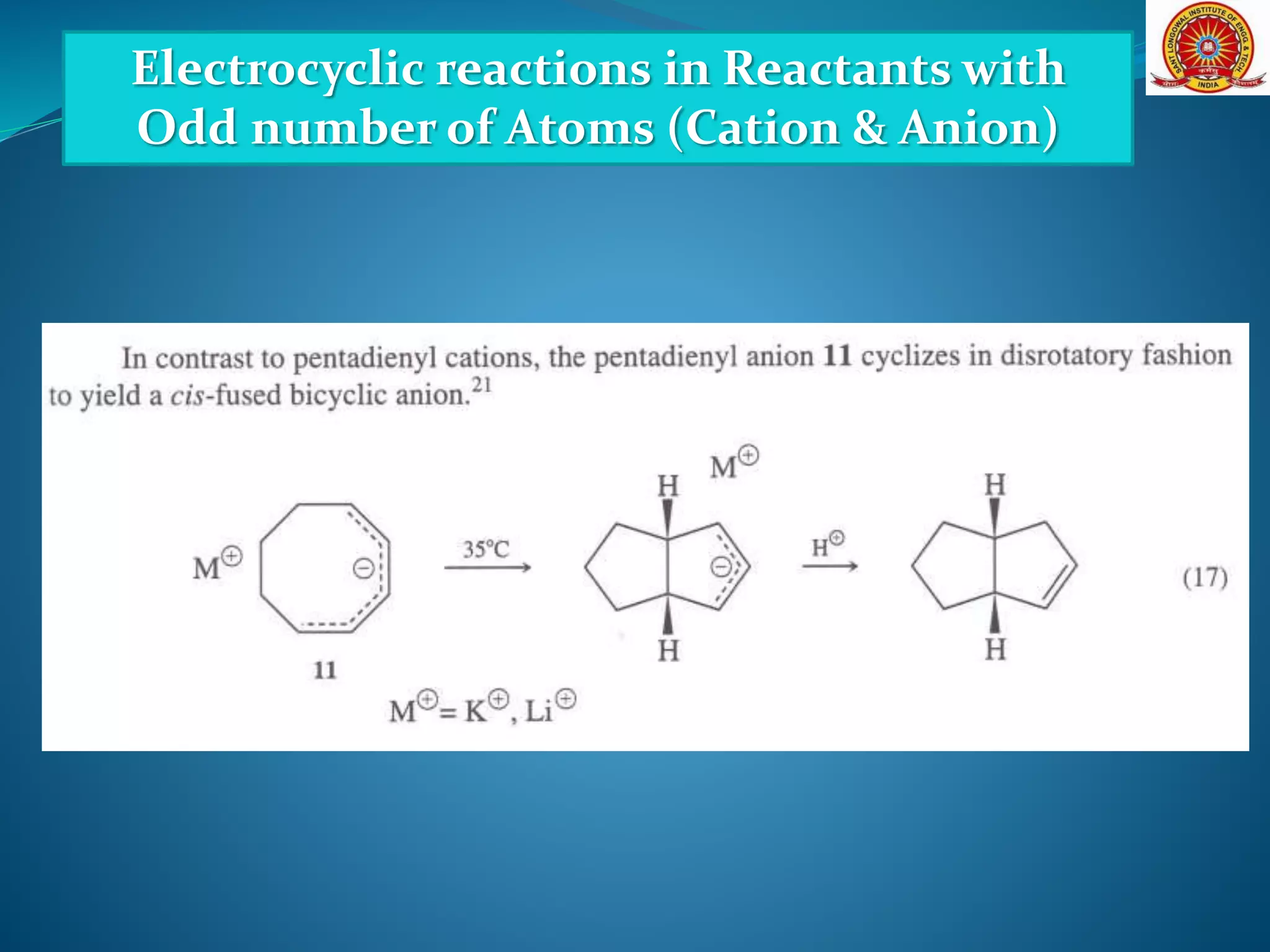
![Electrocyclic reactions in Reactants with
Odd number of Atoms (Cation & Anion)
[Formation and Cyclization of Dipolar Molecules]](https://image.slidesharecdn.com/electrocyclicreactions-200513124943/75/Electrocyclic-reactions-23-2048.jpg)
![Electrocyclic reactions
[Photochemical Cyclizations]](https://image.slidesharecdn.com/electrocyclicreactions-200513124943/75/Electrocyclic-reactions-24-2048.jpg)
![Electrocyclic reactions
[Photochemical Cyclizations]](https://image.slidesharecdn.com/electrocyclicreactions-200513124943/75/Electrocyclic-reactions-25-2048.jpg)
![Electrocyclic reactions
[Photochemical Cyclizations]
STEREOSPECIFIC REACTIONS](https://image.slidesharecdn.com/electrocyclicreactions-200513124943/75/Electrocyclic-reactions-26-2048.jpg)
![Electrocyclic reactions
[Photochemical Cyclizations]
NON-STEREOSPECIFIC REACTIONS
1,3-Butadiene cyclize on photo-irradiation with UV light
with wavelengths above 220 nm to form the predicted
DISROTATORY ring closure products](https://image.slidesharecdn.com/electrocyclicreactions-200513124943/75/Electrocyclic-reactions-27-2048.jpg)
![Electrocyclic reactions
[Photochemical Cyclizations]
NON-STEREOSPECIFIC REACTIONS](https://image.slidesharecdn.com/electrocyclicreactions-200513124943/75/Electrocyclic-reactions-28-2048.jpg)