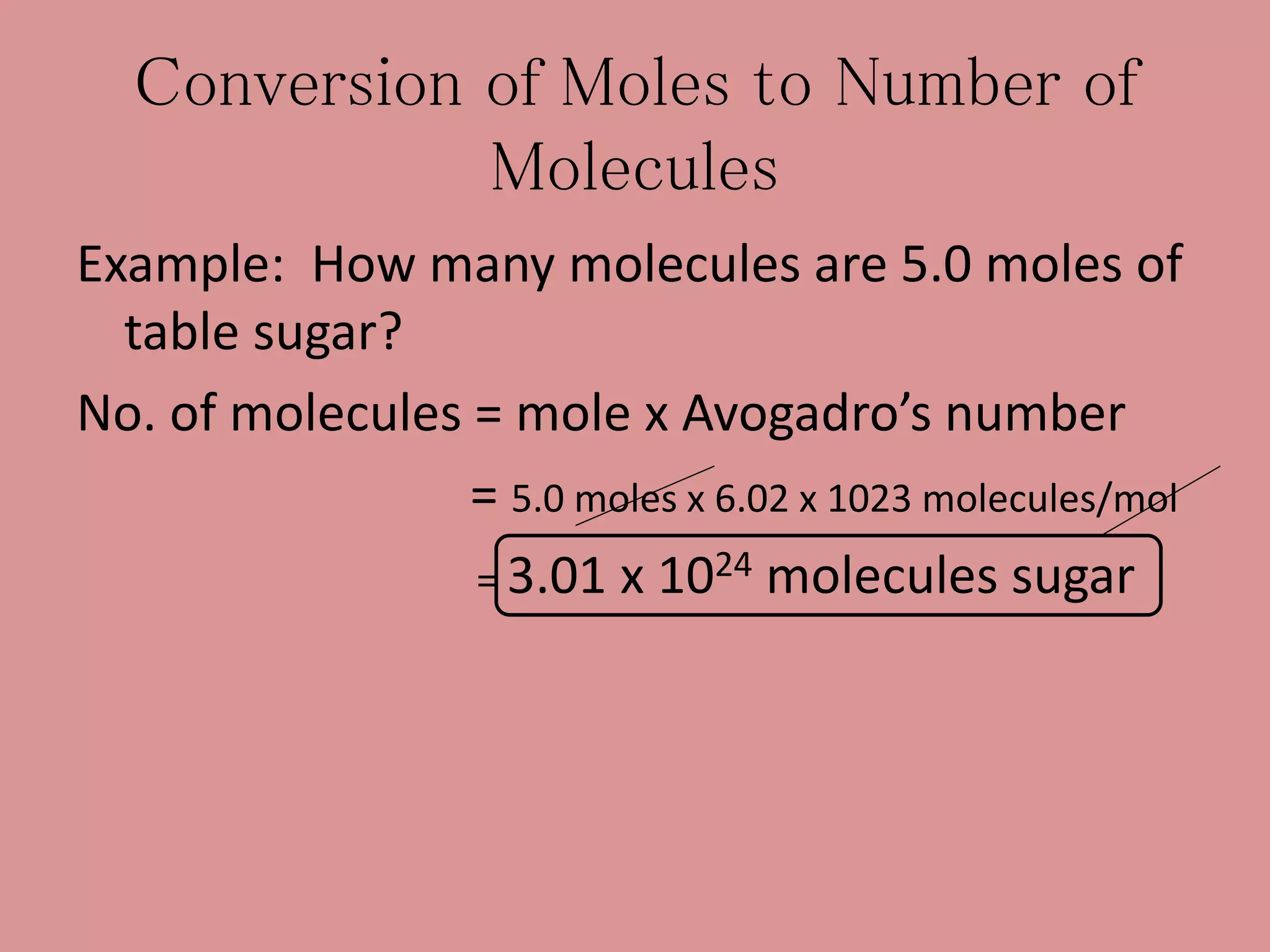
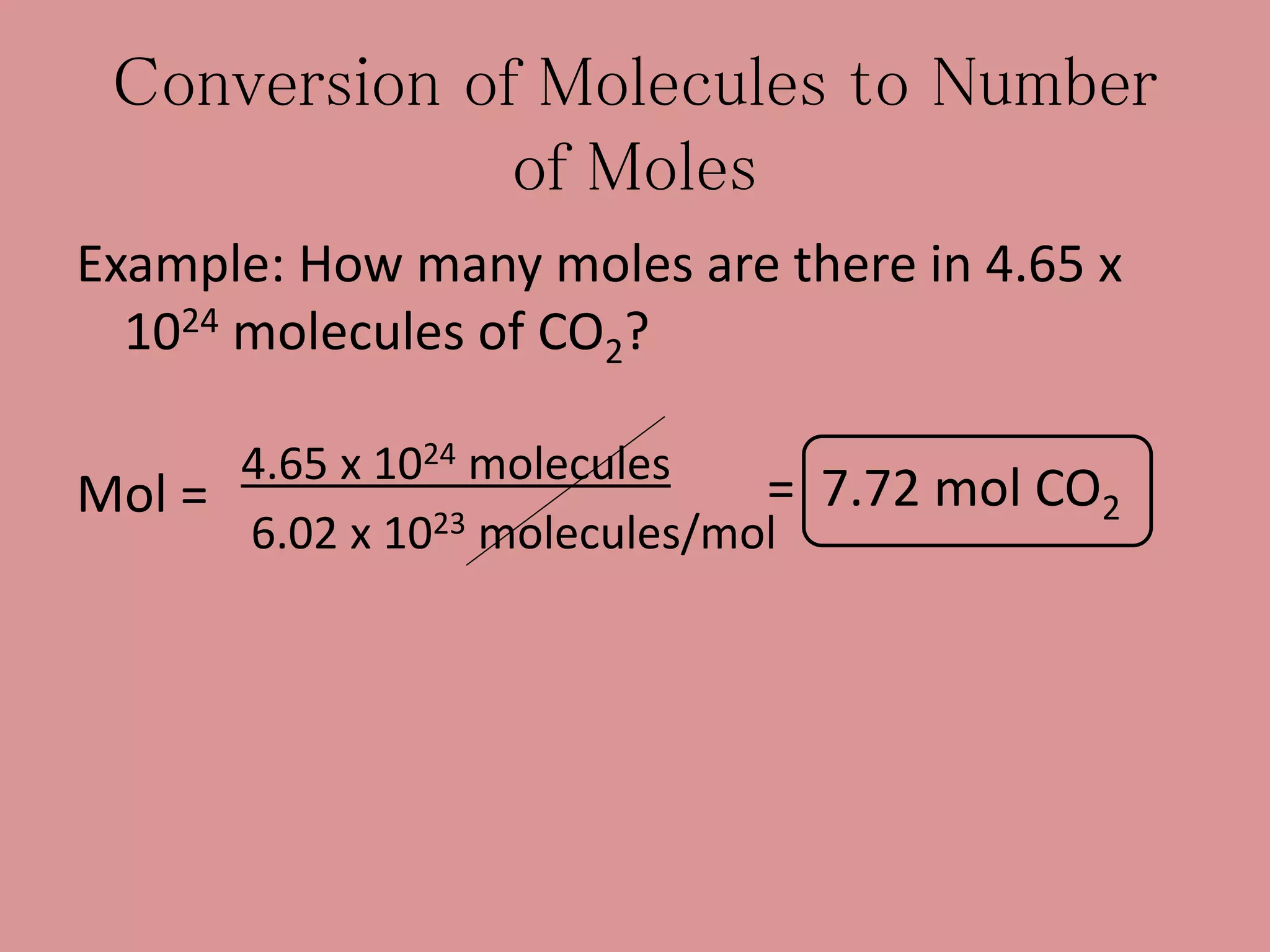
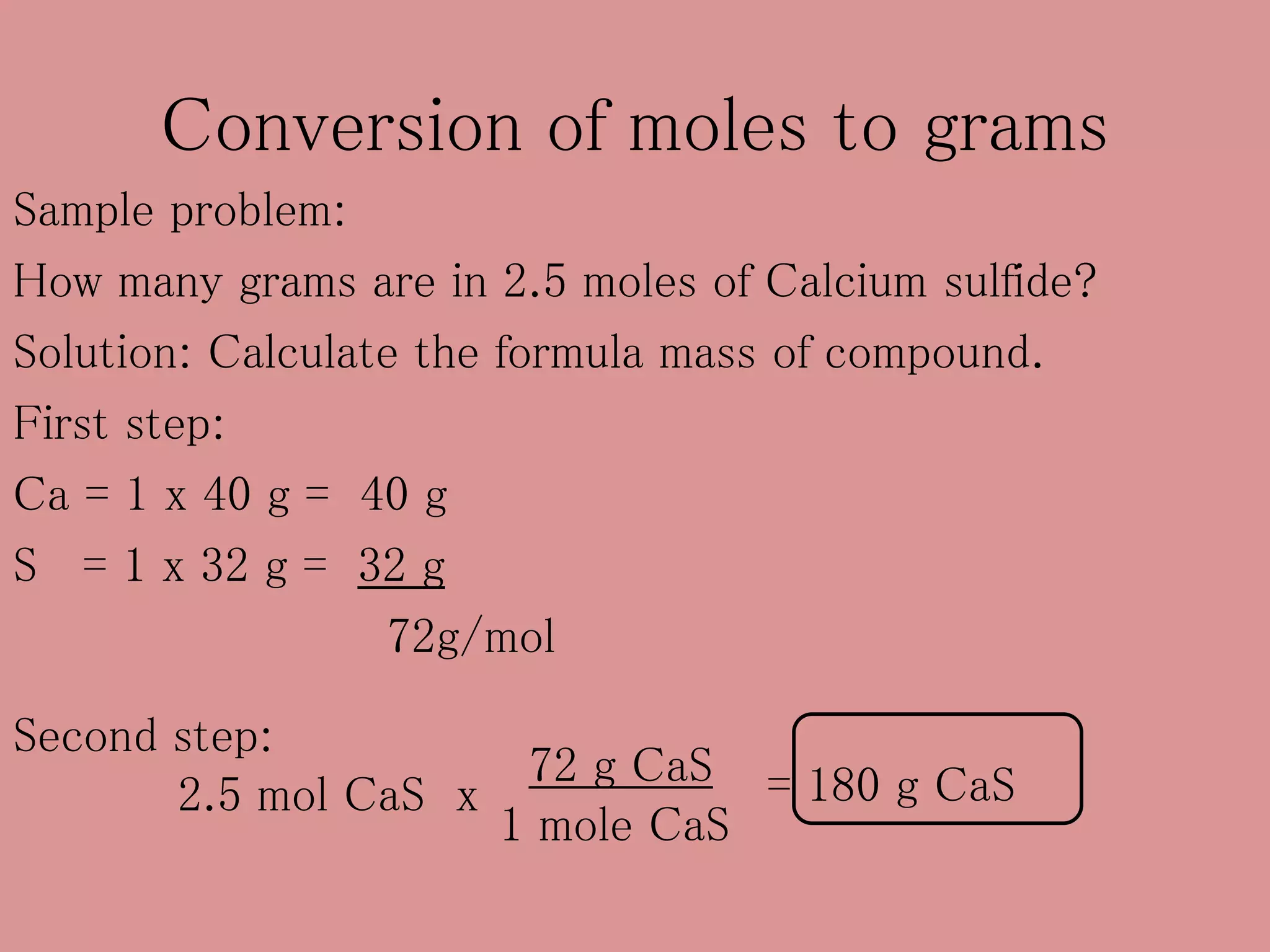
- The mole concept allows chemists to conveniently keep track of large numbers of particles. A mole is defined as 6.02 x 1023 particles, whether atoms, molecules, ions, etc.
- The formula mass (or molar mass) of a compound is the sum of the atomic masses of each element in its chemical formula. It has units of grams per mole (g/mol).
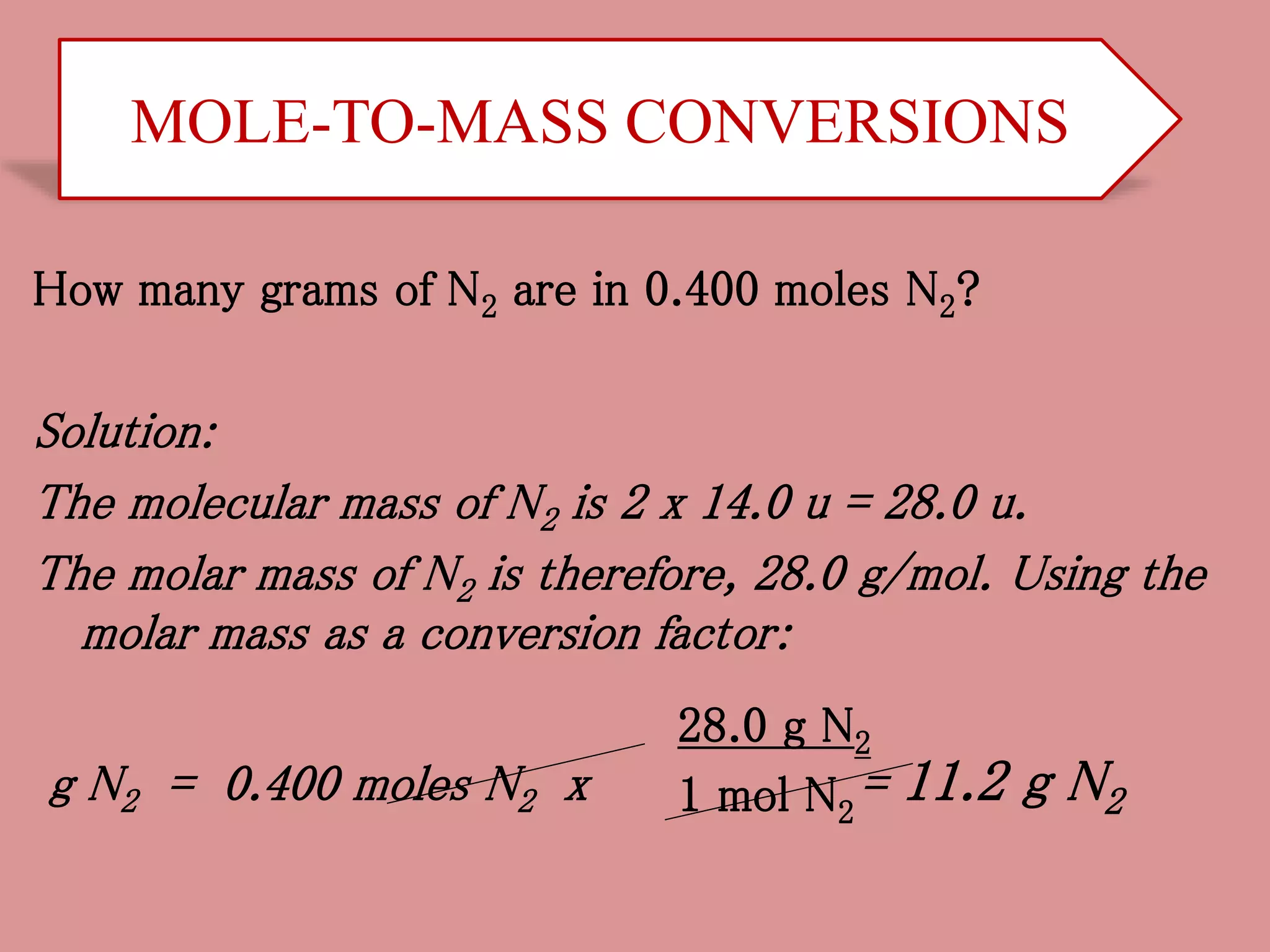
- Calculations involving moles, mass, particles and formula mass allow conversions between the microscopic and macroscopic scales in chemistry.


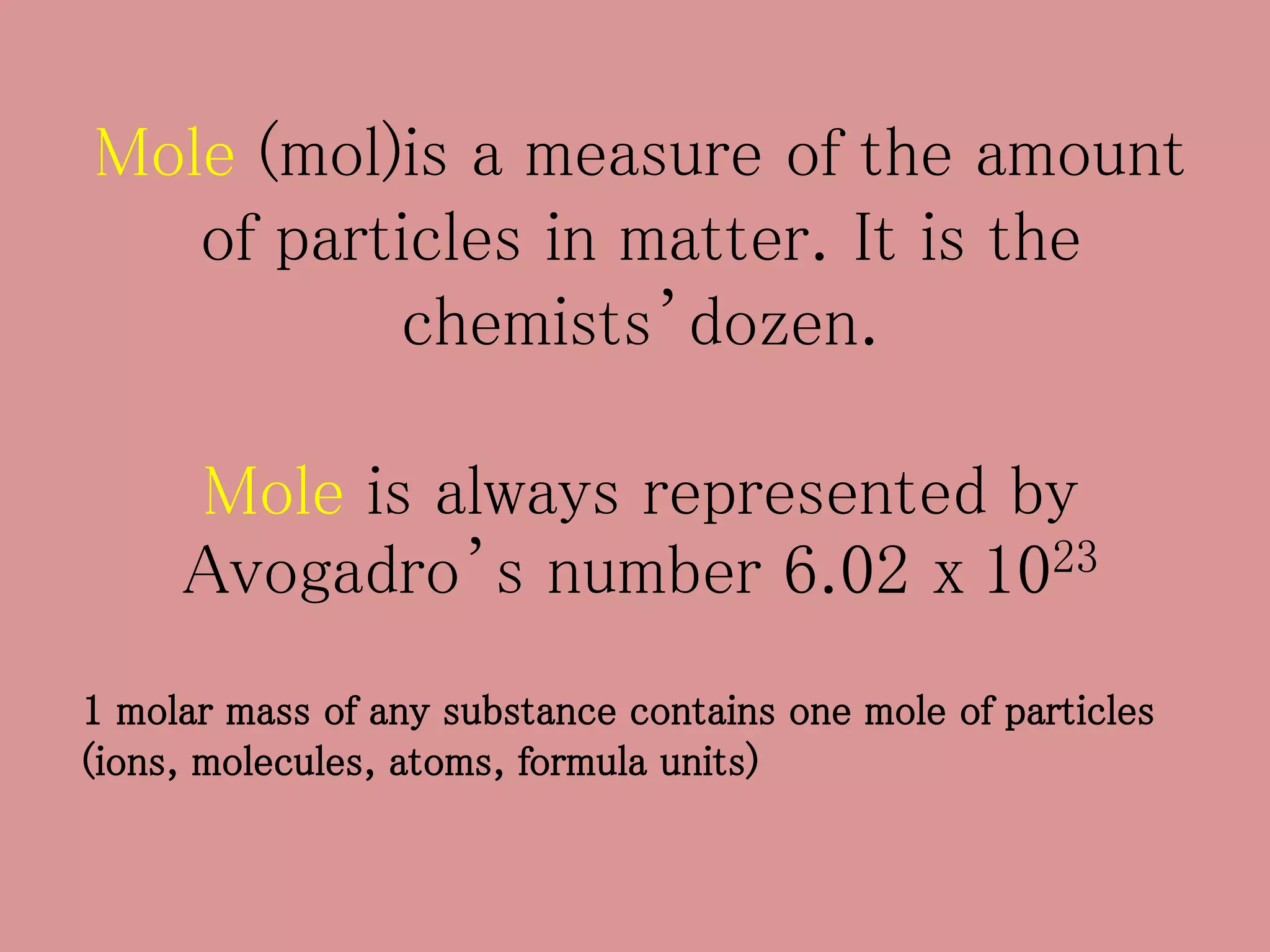


![Calculate (a) the molecular mass of nitrogen
dioxide (NO2), an amber colored gas that is a
constituent of smog, and (b) the formula mass
of ammonium sulfate [(NH4)2SO4] a fertilizer
commonly used by home gardeners.
CALCULATING MOLECULAR MASS](https://image.slidesharecdn.com/mole-concept-171018090606/75/Mole-Concept-6-2048.jpg)
![1. Calculate the formula mass of (a) sodium
azide (NaN3) used in automobile airbags, and
(b) phosphoric acid (H3PO4)
2. Find the formula mass of (a) para-
dichlorobenzene (C6H4Cl2)used as a moth
repellent, and (b) calcium dihydrogen
phosphate [Ca(H2PO4)2], used as a mineral
supplement in foods.
PRACTICE:](https://image.slidesharecdn.com/mole-concept-171018090606/75/Mole-Concept-7-2048.jpg)


![Find the formula mass of the following compounds:
1. C6H12O6 6. AlPO4
2. (NH2Cl)3MnO4 7. ZnCO3
3. NH4 8. KOH
4. F2 9. Al2(SO4)3
5. H2O2 10. [(Fe2O3)(SO2)]
SEATWORK:](https://image.slidesharecdn.com/mole-concept-171018090606/75/Mole-Concept-11-2048.jpg)