1. A mole is defined as the amount of substance containing 6.02 x 1023 particles and can refer to atoms, molecules, or ions.
2. The mole is the unit for quantifying amount of substance, with the symbol "mol".
3. The number of particles in a given number of moles can be calculated by multiplying the moles by Avogadro's number, and the moles for a given number of particles is calculated by dividing the particles by Avogadro's number.

![Number of Mole and Number of Particles
1. We have just learn that, mole is a quantity, and it is equal to 6.02 x 1023.
The number 6.02 x 1023 is called the Avogadro constant.
2. Therefore, if we are given the number of mole of substance, and asked
to find the number of particles (atoms, molecules or ions) in it, we
multiply the number of mole by the Avogadro constant.
3. Likewise, if we are given the number of particles, and asked to find the
number of mole of the particles, we divide the number of particles by
the Avogadro constant.
Example
Which contains more atoms, 1 mol of helium or 1 mol of uranium? Which has
a greater mass? [ RAM: He=4; U=238 ]
Answer:
1 mol of helium and 1 mol of uranium has equal number of atoms.
The mass of one Uranium atom is greater than the mass of one helium atom.
Example
Find the number of atoms in 2.5 mol of gold.
Answer:
Number of atoms
= Number of mole x Avogadro constant
= 2.5 x 6.02 x 1023 = 1.505 x 1024
Example
How many moles of magnesium that contain 2.76 x 1023 of magnesium atom?
Answer:
Number of mole
= Number of atoms ÷ Avogadro constant](https://image.slidesharecdn.com/conceptofmole-140224101818-phpapp02/75/Concept-of-mole-2-2048.jpg)

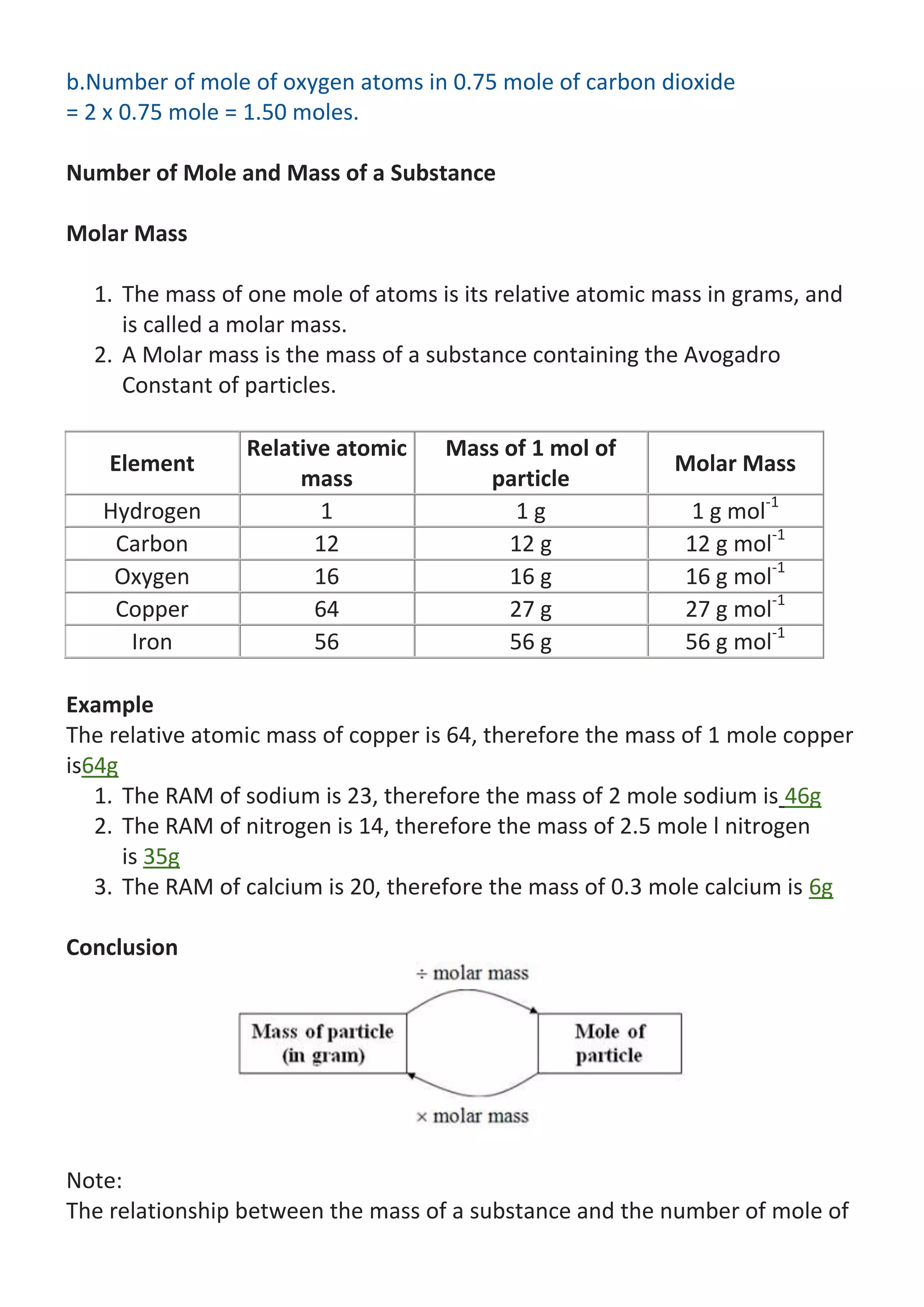
![the particles in the substance can be summarised by using the following
equation:
n=m / Molar Mass
If you are given the mass of substance and asked to find the number of mole
of the substance (or vice versa), the problem can be solved by using this
equation.
Example:
Find the number of mol of atoms in 4.6g sodium [Relative atomic mass:
Na=23]
Answer:
Number of mole,
N=4.6 / 23=0.2mol
Example
How many moles of each substance are there in 191 g NaOH [Relative atomic
mass: Na=23, O=16, H=1]
Answer:
Relative Formula Mass of NaOH = 23 + 16 + 1 = 40
Number of mole of 191g NaOH,
n=191 / 40=4.775mol
Question
What is the mass of 7.12 molNaI. [Relative atomic gas: Iodine = 131; Sodium =
23]
Answer:
The relative formula mass of NaI = 23 + 131 = 154.
The mass of 7.12 molNaI
= Number of mole x Relative Formula Mass of NaI
= 7.12 x 154
= 1096.48g](https://image.slidesharecdn.com/conceptofmole-140224101818-phpapp02/75/Concept-of-mole-5-2048.jpg)
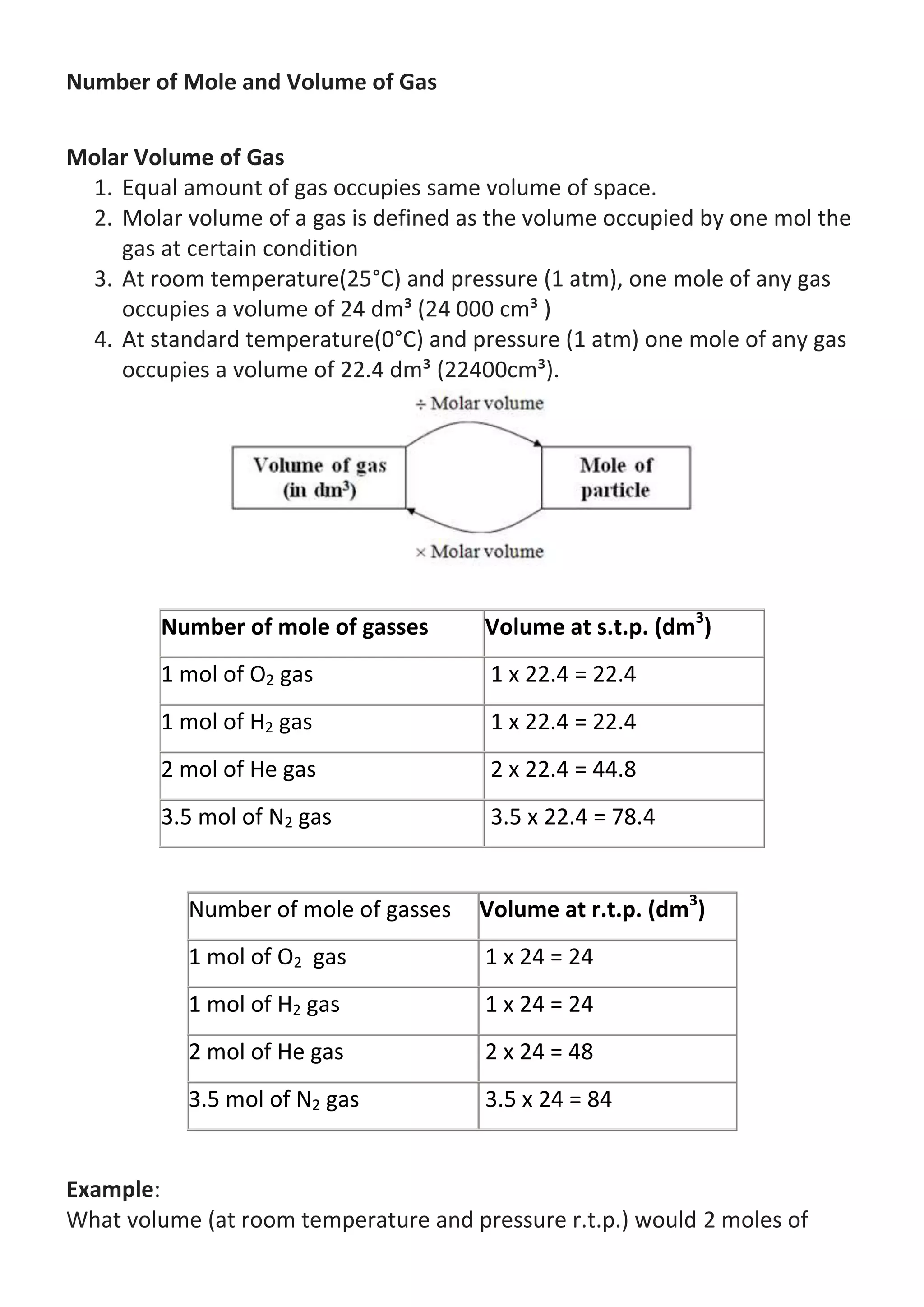
![oxygen gas occupy? (Molar Gas Volume at r.t.p. = 24 dm³)
Answer:
Volume of gas = 2 x 24 = 48 dm³
Example:
A sample of ozone gas has a volume of 960cm³ at room temperature and
pressure. Find the number of mole of the ozone. [Molar volume at r.t.p. =
24.0dm³]
Answer:
Number of mole=960cm3 / 24000cm3=0.04 mol](https://image.slidesharecdn.com/conceptofmole-140224101818-phpapp02/75/Concept-of-mole-7-2048.jpg)