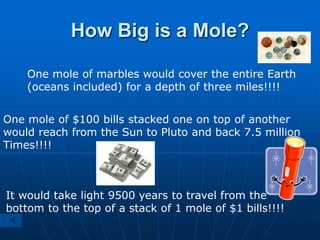
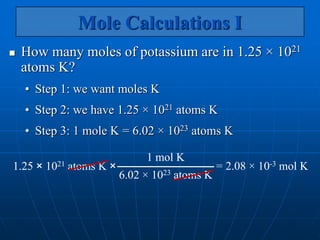
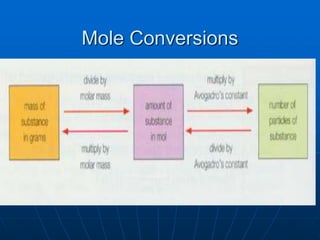
The document discusses the mole concept in chemistry. It defines the mole as the amount of substance that contains as many elementary units (atoms, molecules, ions) as there are atoms in exactly 12 grams of carbon-12. The number of elementary units in one mole is Avogadro's number, which is approximately 6.022x1023. Examples are given to illustrate that one mole of any substance will contain this number of particles, though the masses in grams will vary depending on the molar masses of the substances. The mole can be used to convert between the number of particles, moles, and mass in grams for calculations involving chemical substances.