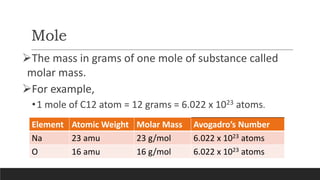
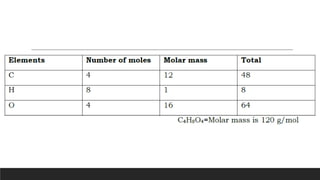
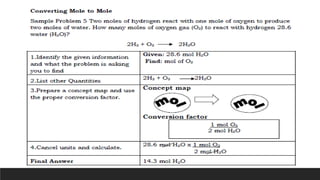
The document discusses the mole, which is defined as 6.022x1023 particles and is used as a unit to describe large numbers of atoms or molecules. It explains that the molar mass of a substance is the mass in grams of one mole of that substance, and provides examples of calculating the number of moles and particles for given masses of various elements and compounds. The document also demonstrates how to use the mole concept to convert between mass and number of particles.