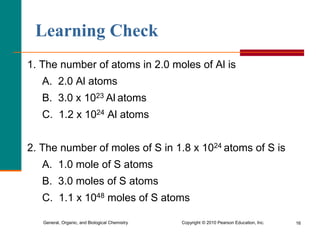
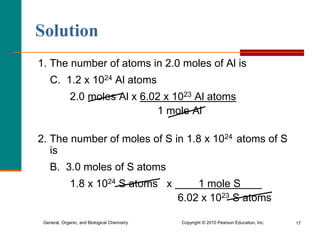
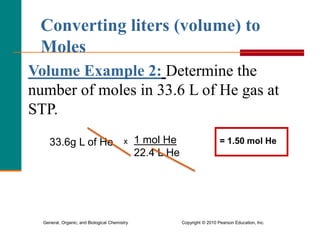
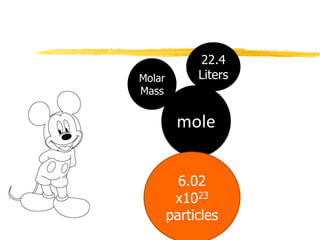
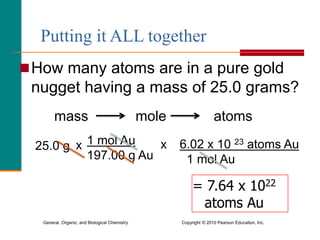
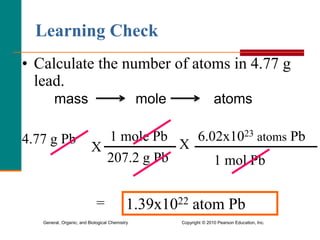
1. The document discusses different units used to measure amount of substance, including moles, grams, particles, and liters at STP.
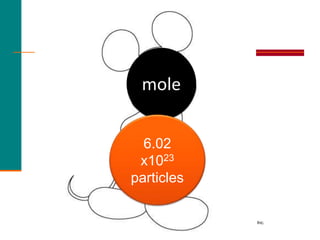
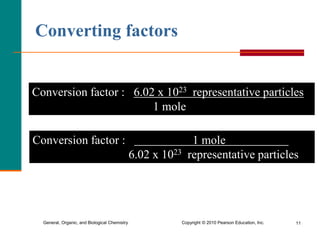
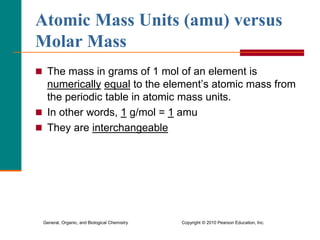
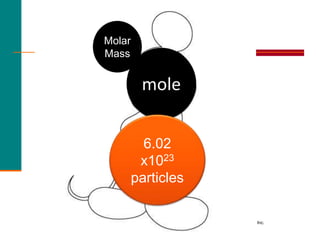
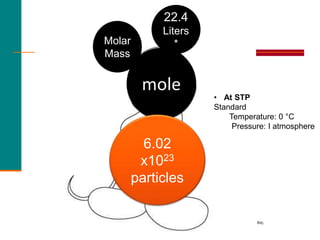
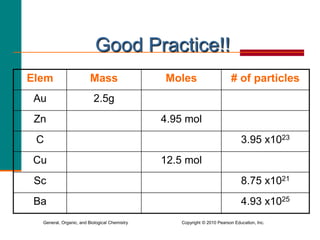
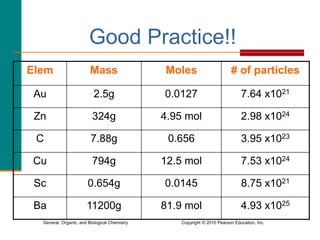
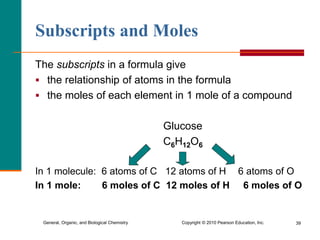
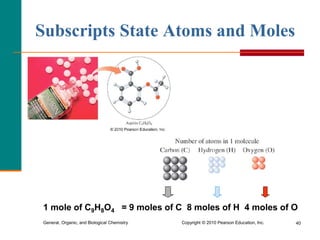
2. A mole is defined as 6.02 x 1023 representative particles and is used to quantify atoms, molecules, ions, and formula units.
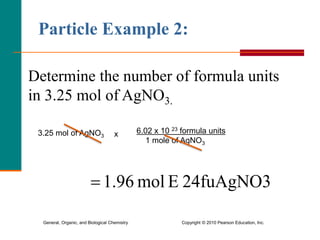
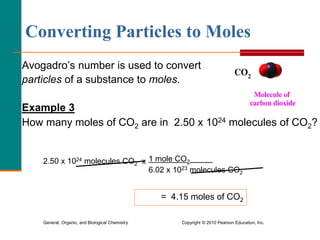
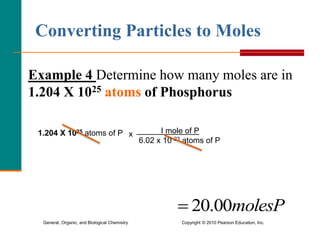
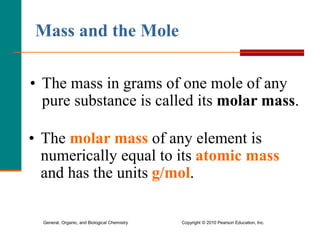
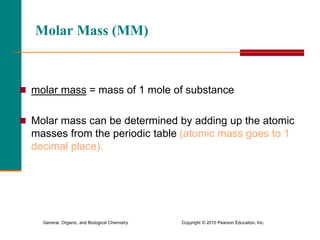
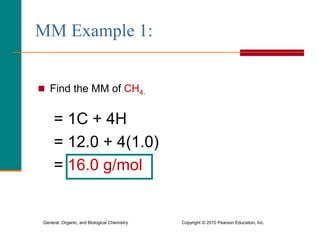
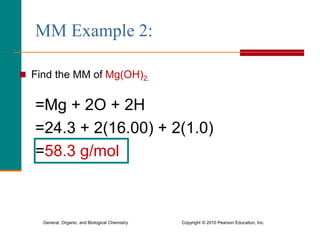
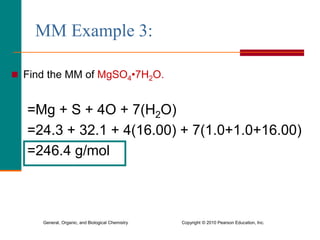
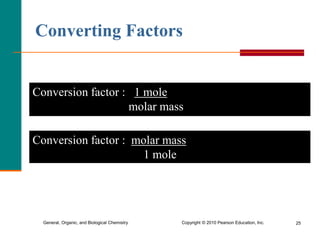
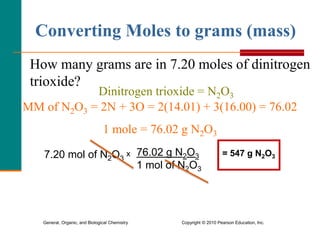
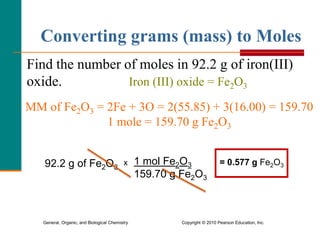
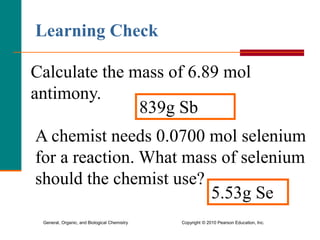
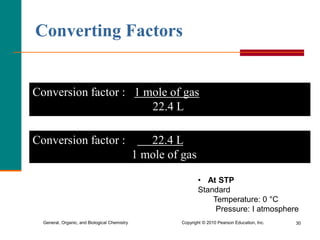
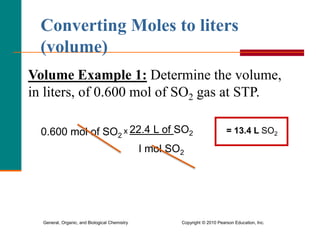
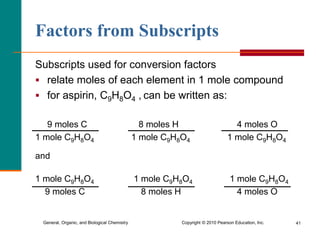
3. Conversions between moles, mass in grams, number of particles, and volume in liters can be performed using appropriate conversion factors and molar masses.