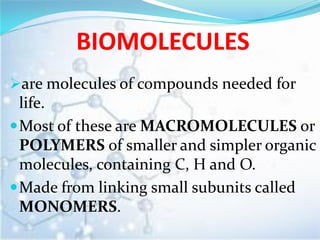
Biomolecules are organic compounds needed for life. They include carbohydrates, lipids, proteins, and nucleic acids. Carbohydrates like starch, glycogen, and cellulose are used for energy storage. Lipids such as fats and oils store energy and act as cell membrane components. Proteins are made of amino acids and have structural and mechanical functions. Nucleic acids like DNA and RNA control cell functions and carry genetic information.