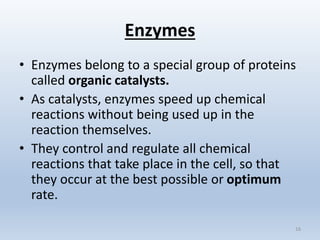
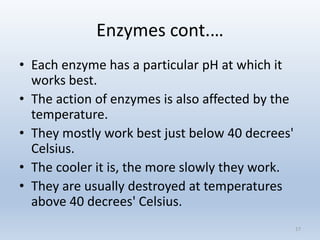
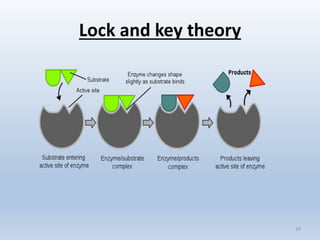
The document covers the fundamental concepts of organic compounds, focusing on carbohydrates, lipids, proteins, enzymes, nucleic acids, and vitamins. It explains the composition, structure, and functions of these biomolecules, along with their importance in biological processes. Additionally, it addresses the role of enzymes in chemical reactions and how DNA and genes govern traits in organisms.














![Activity
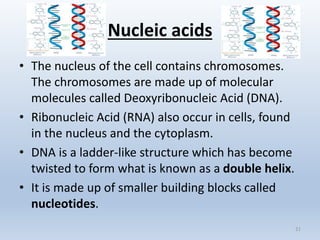
1. What are the organic molecules that make up
chromosomes? [1]
2. Building blocks of DNA?[1]
3. The twisted ladder-like appearance of DNA.[1]
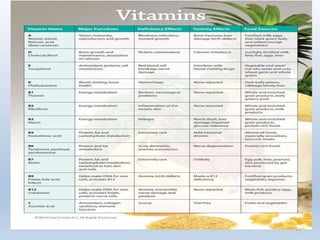
Name the vitamin that applies to each of the following
functions, sources or deficiencies.[5]
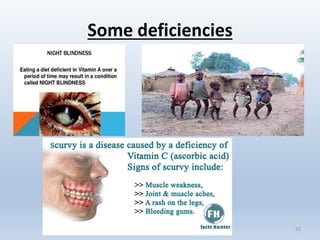
• Scurvy.
• Rickets.
• Night- blindness.
• Essential for the correct functioning of the eye.
• Abundant in citrus fruits.
26](https://image.slidesharecdn.com/slideshares-170825110151/85/Life-sciences-grade-10-26-320.jpg)