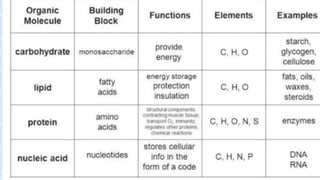
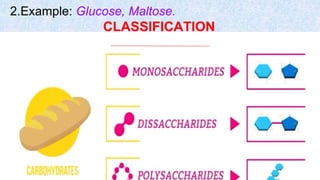
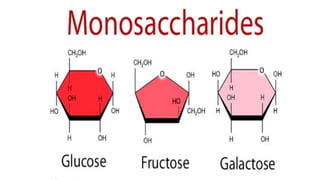
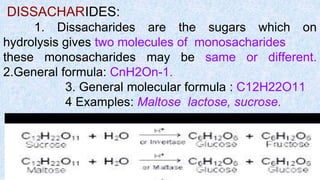
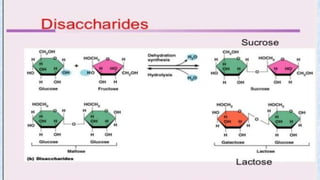
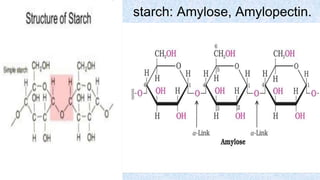
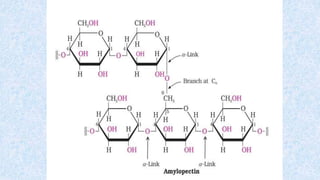
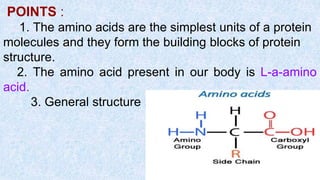
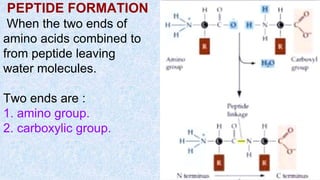
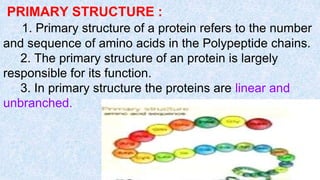
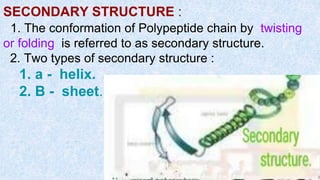
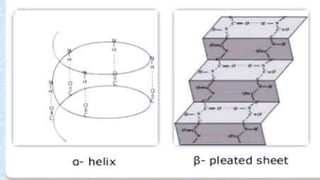
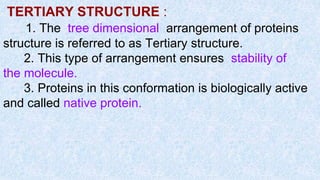
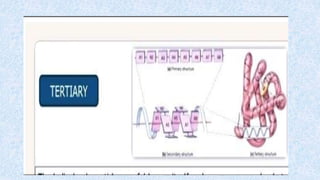
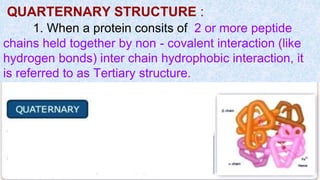
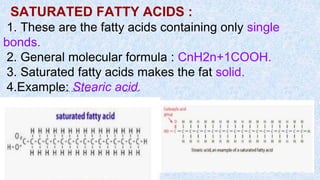
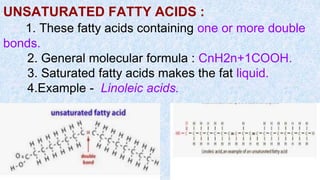
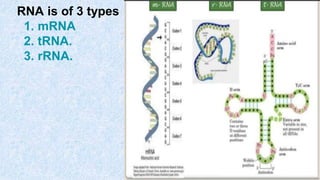
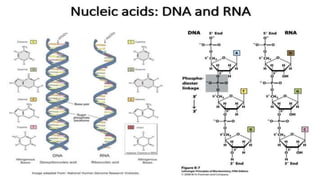
The document provides an in-depth overview of biomolecules, classifying them into carbohydrates, amino acids and proteins, lipids, and nucleic acids, explaining their structures, functions, and biological significance. It discusses the composition and types of carbohydrates, the building blocks of proteins, and the characteristics and roles of lipids including their classification and functions. Additionally, the text delves into nucleic acids, specifically DNA and RNA, their role in genetic information storage, and the importance of each type of biomolecule in living organisms.