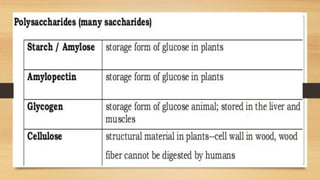
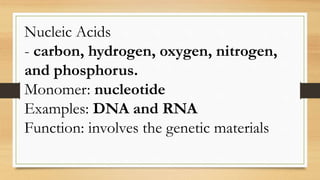
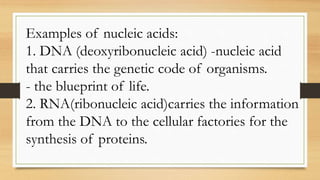
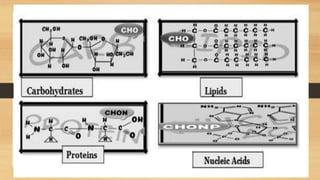
The document outlines the four main types of biological macromolecules: carbohydrates, lipids, proteins, and nucleic acids, detailing their structures, classifications, and functions. Carbohydrates are categorized into simple and complex sugars, lipids are classified as triglycerides and unsaturated or saturated fats, proteins consist of amino acids, and nucleic acids are made of nucleotides storing genetic information. Each biomolecule plays a vital role in various biological processes, such as energy storage, structural support, and the transfer of genetic information.