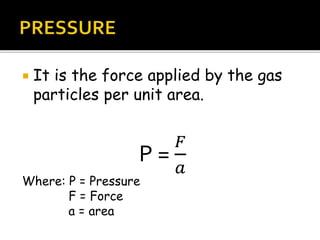
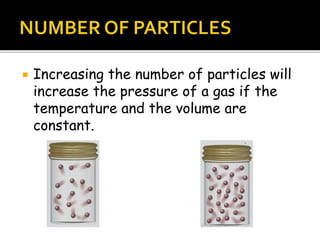
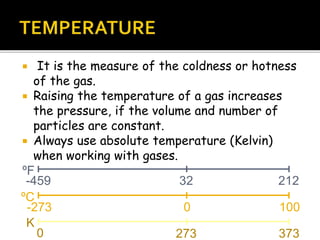
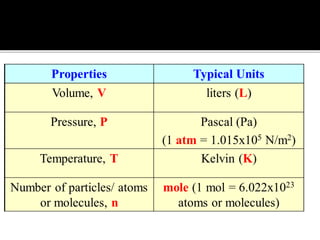
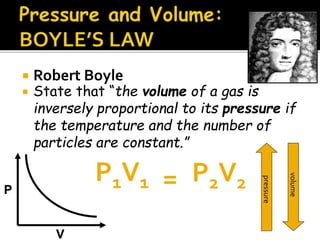
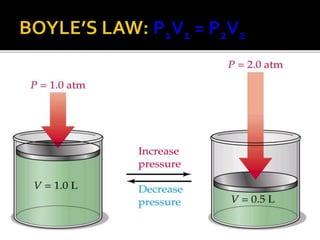
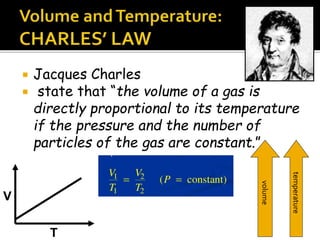
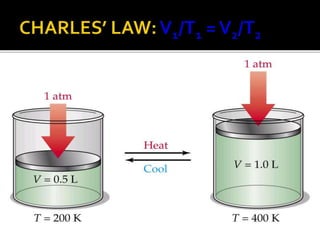
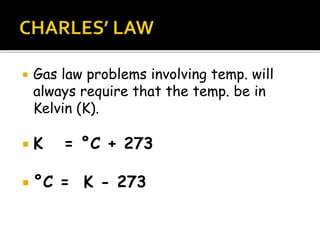
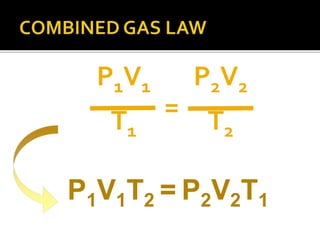
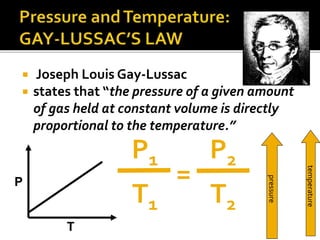
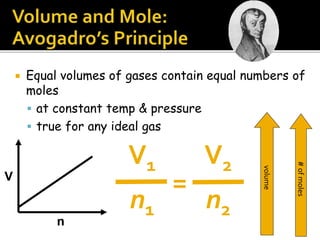
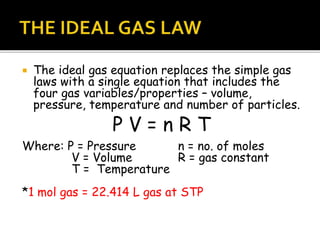
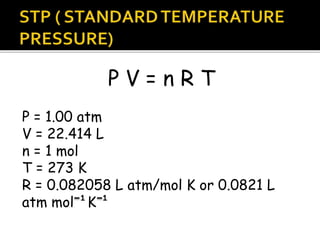
The document outlines key concepts related to gas behavior, including Boyle's Law, Charles's Law, and Gay-Lussac's Law, along with the ideal gas equation. It explains how gases behave under various conditions of pressure, volume, and temperature, and includes problem-solving examples to illustrate these laws. Additionally, it discusses Avogadro's principle regarding the relationship between gas volume and the number of moles at constant temperature and pressure.