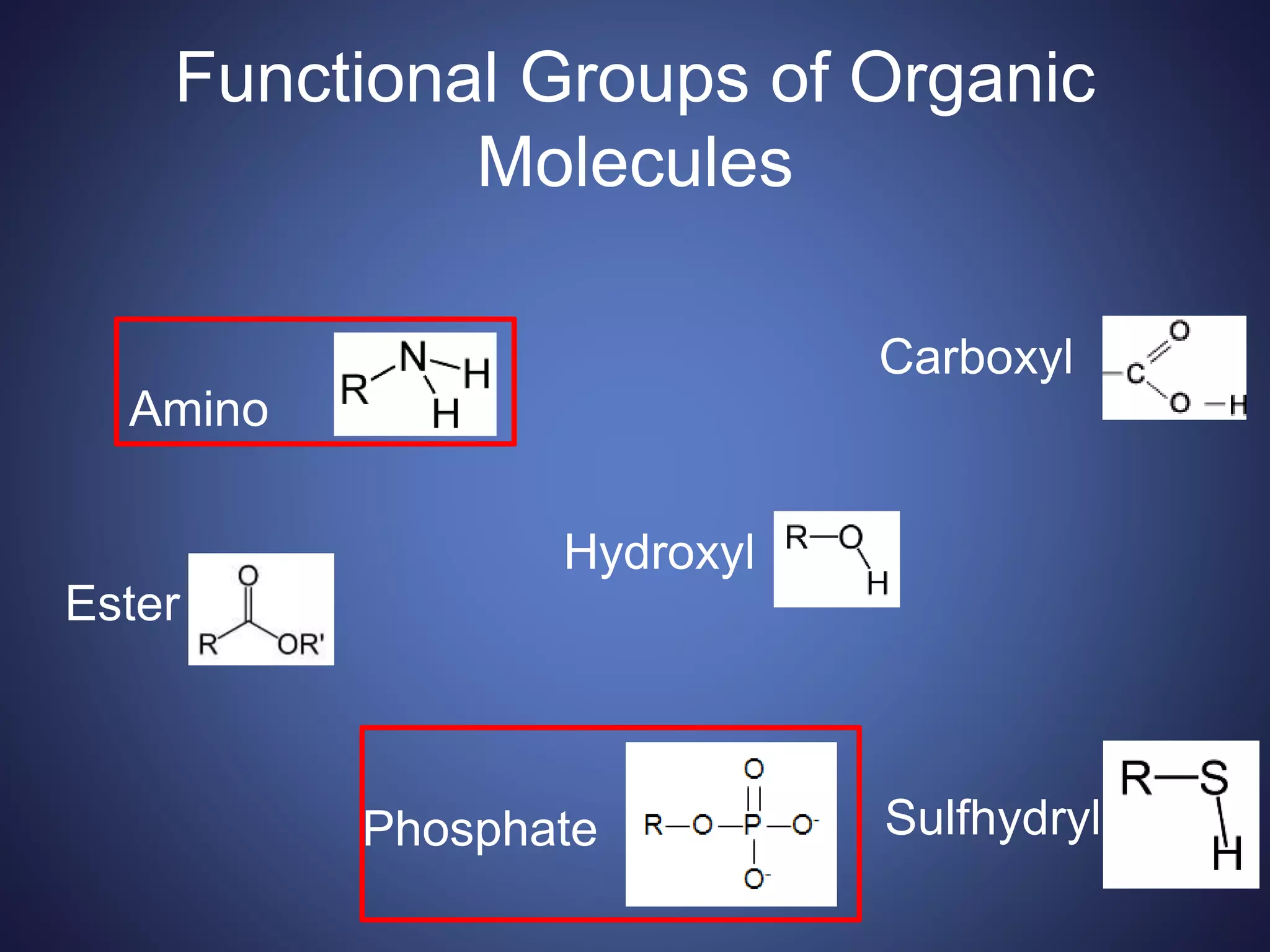
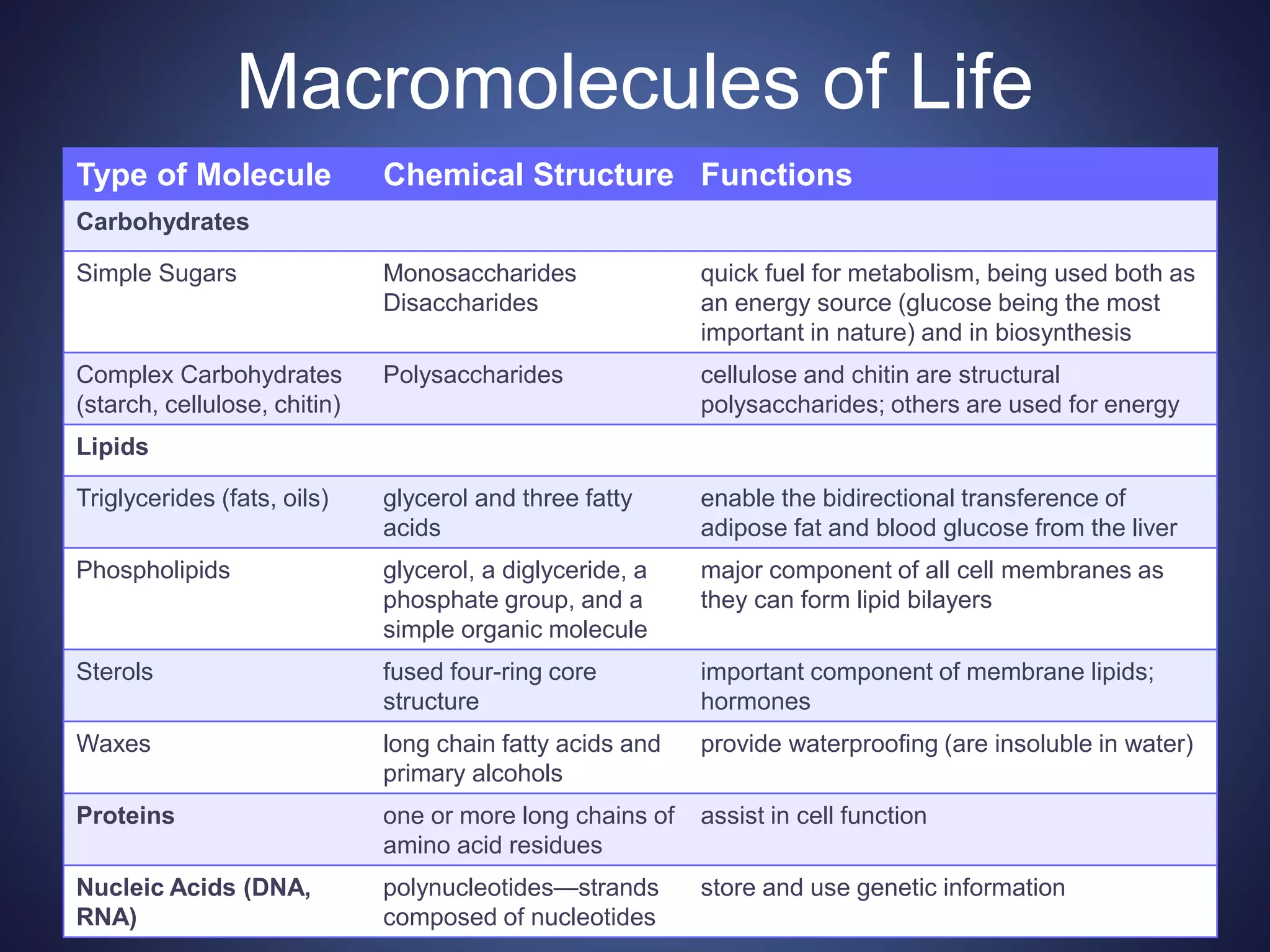
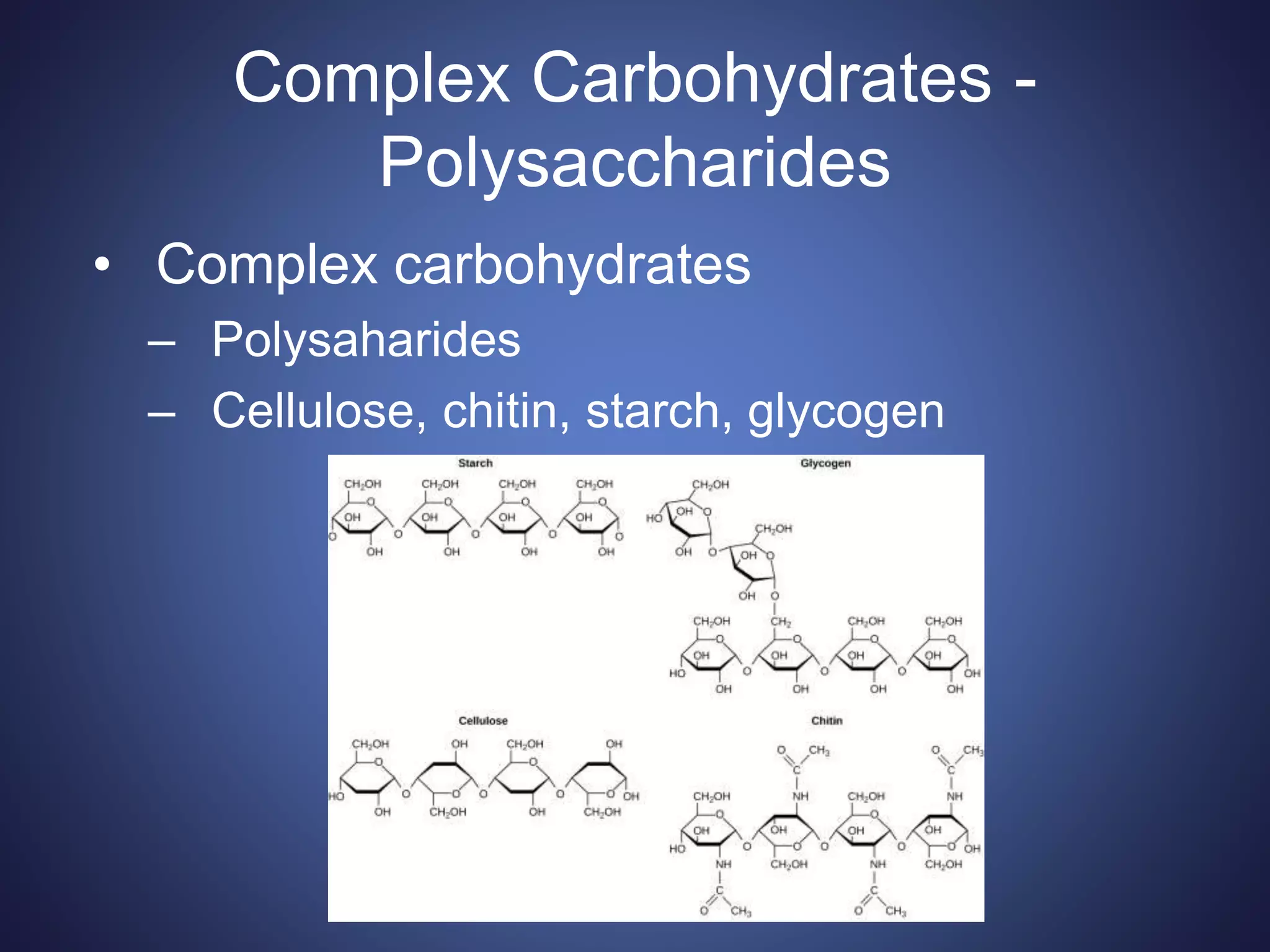
This document summarizes the key organic molecules found in living things: carbohydrates, lipids, proteins, and nucleic acids. It describes their main functions and chemical structures. Carbohydrates include sugars that serve as fuels or provide structure. Lipids are hydrophobic and serve roles in energy storage, insulation, and signaling. Proteins are made of amino acids and perform most chemical reactions in cells. Nucleic acids like DNA and RNA store and transmit genetic information through sequences of nucleotides.