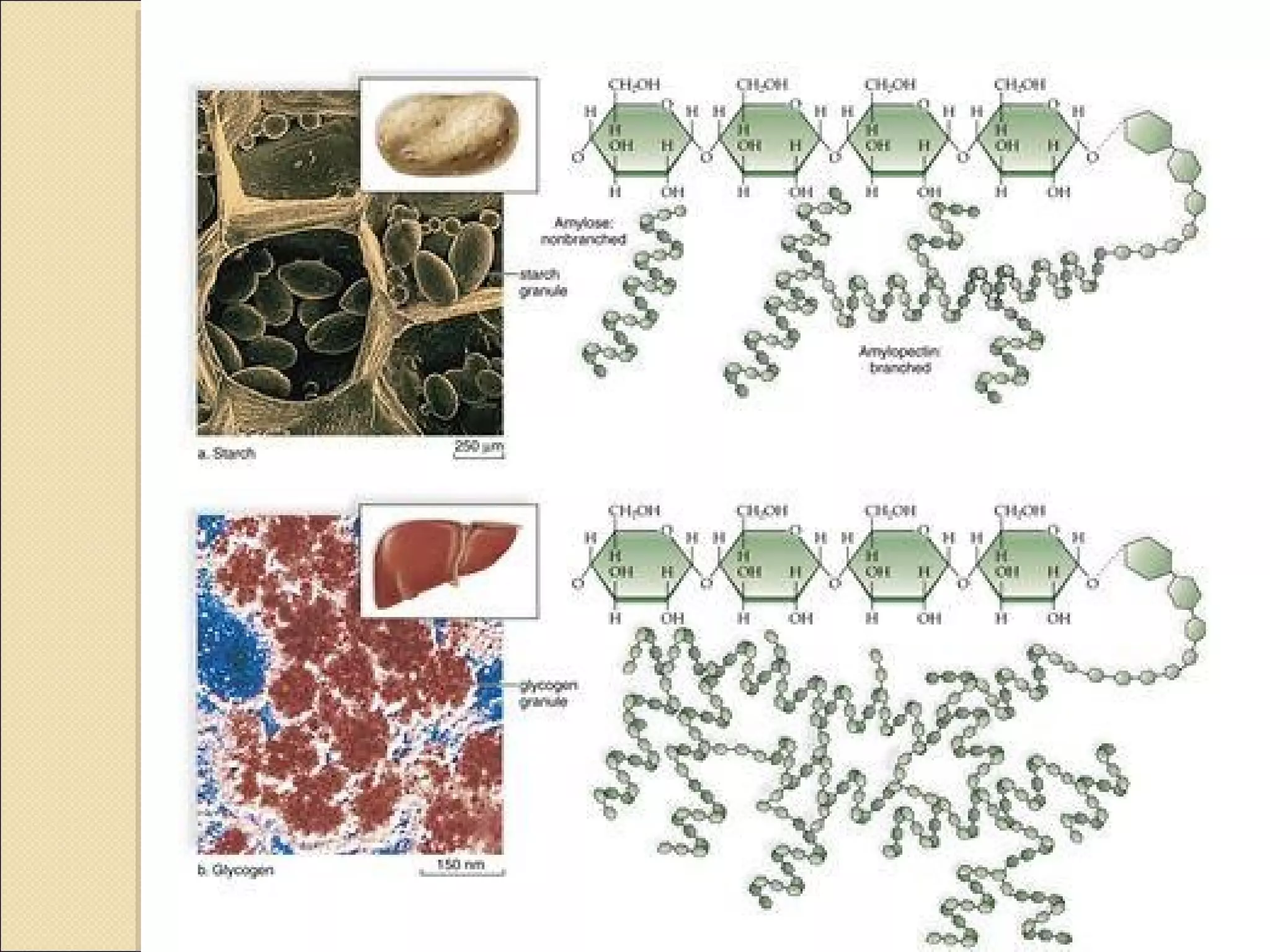
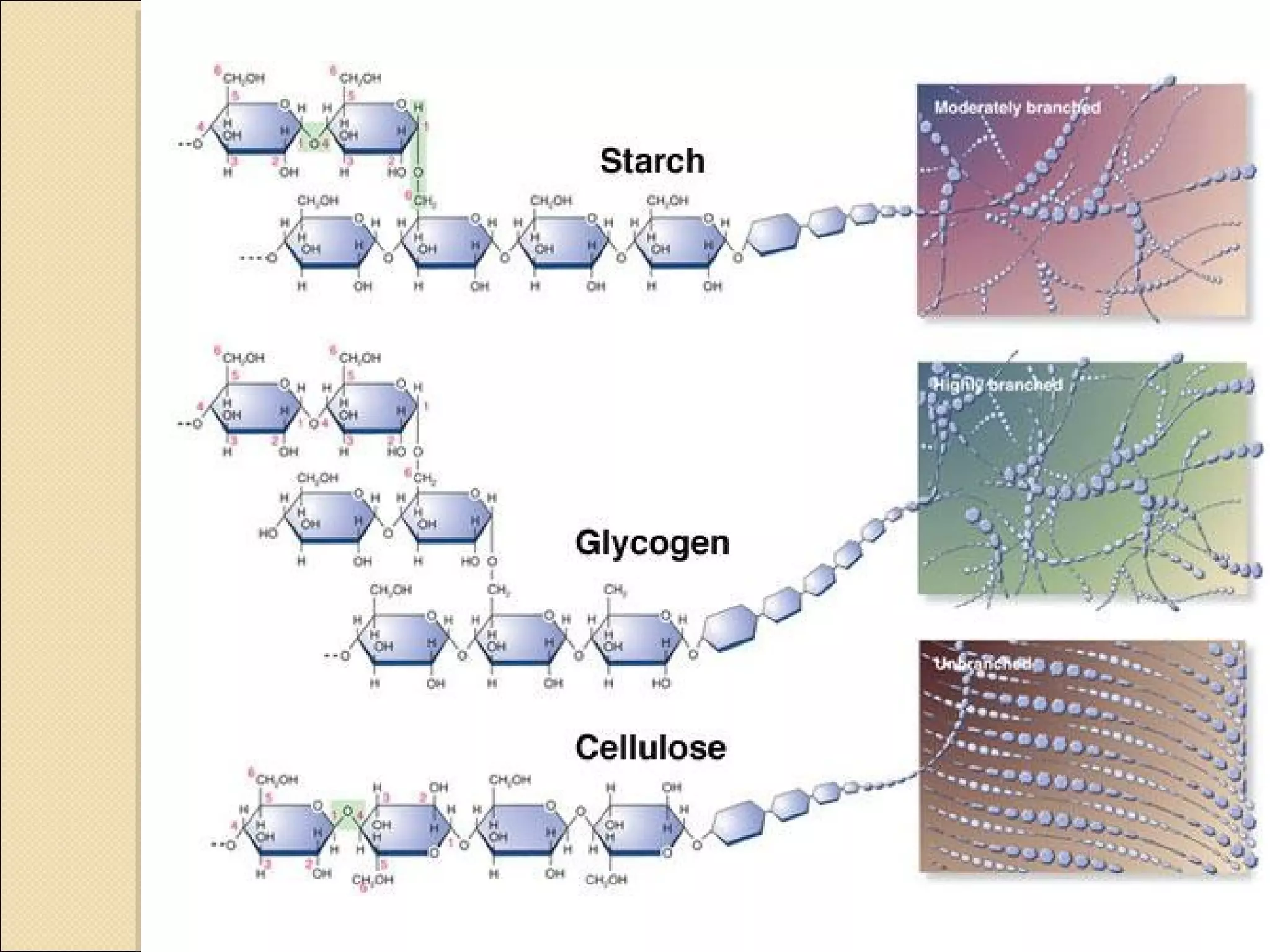
This document discusses biological molecules and biomolecules. It notes that the four most important elements that make up living things are carbon, hydrogen, oxygen, and nitrogen. Carbon is particularly important as it can form many different structures and molecules due to its ability to form up to four bonds. Biomolecules are organic molecules found in living organisms, including macromolecules like carbohydrates, proteins, lipids and nucleic acids. The document then discusses specific biomolecules like carbohydrates, lipids, proteins and water in more detail.