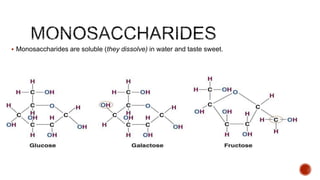
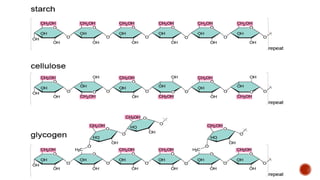
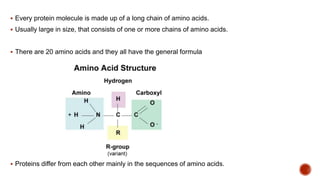
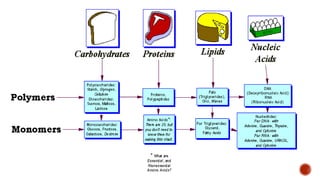
Biomolecules include carbohydrates, lipids, proteins and nucleic acids. Carbohydrates include monosaccharides like glucose that join to form disaccharides like sucrose or polysaccharides like starch for storage. Lipids are insoluble in water, made of fatty acid esters, and serve as energy stores. Proteins are made of amino acid chains and perform important functions like catalysis and transport.