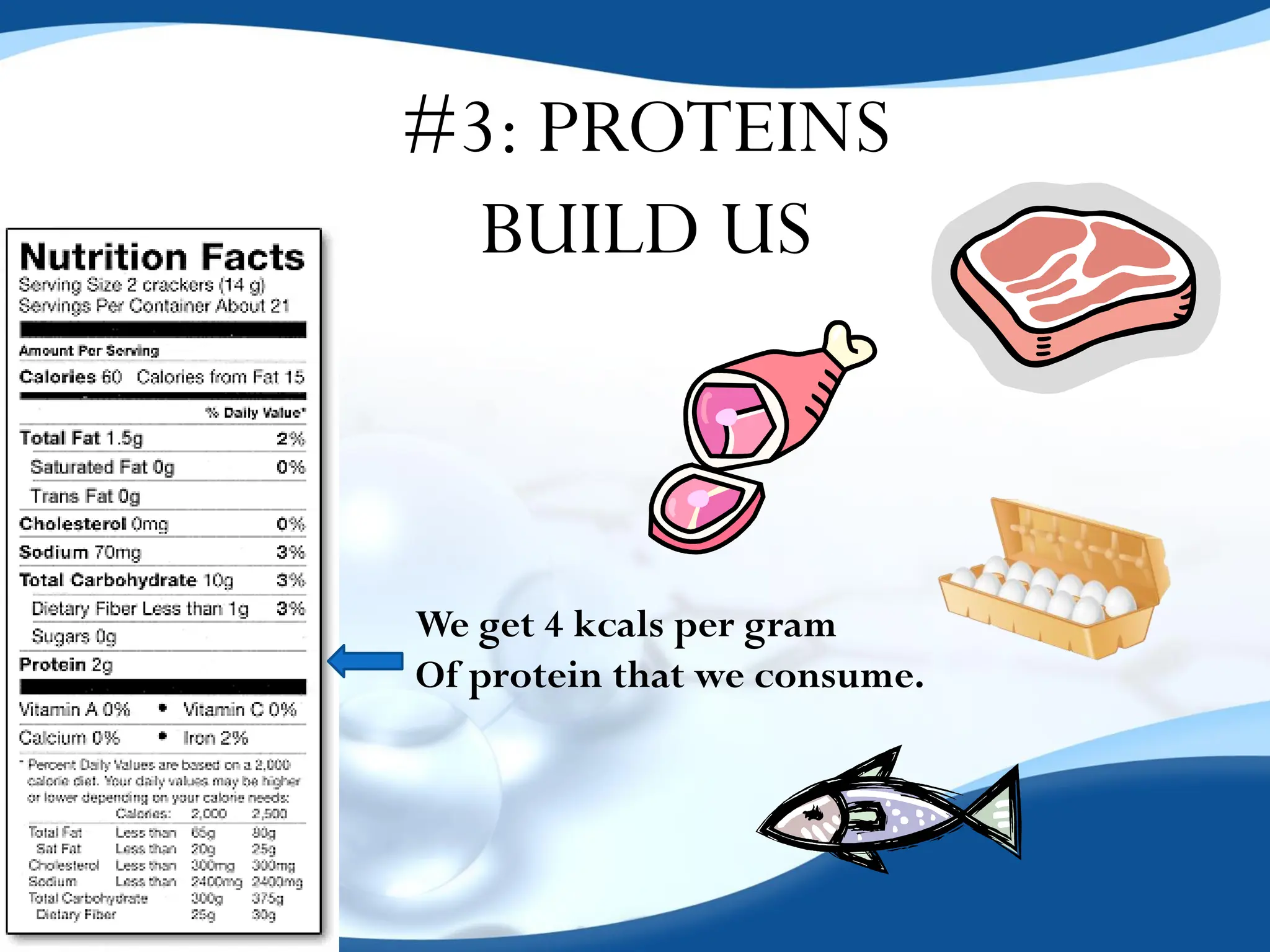
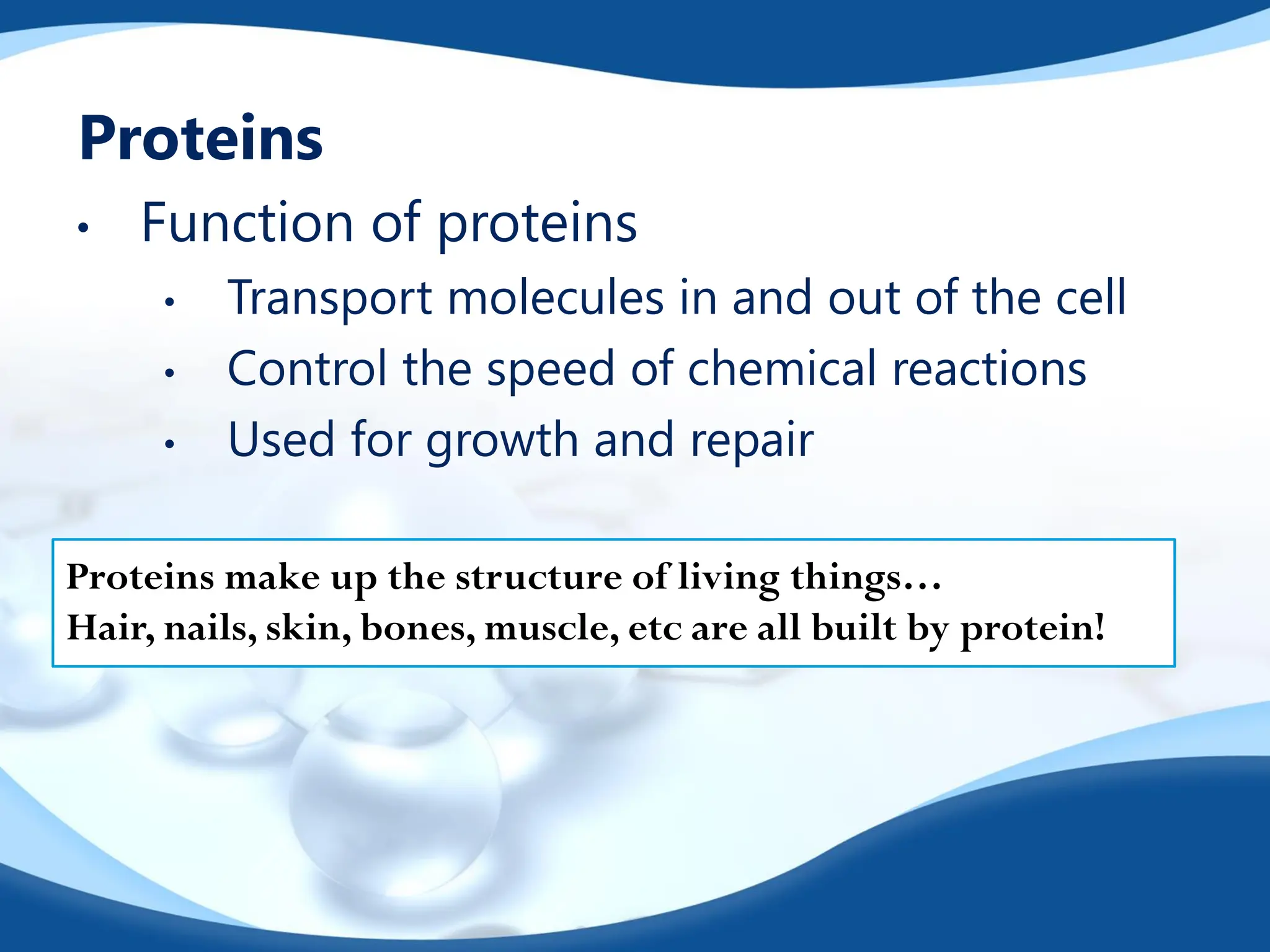
The document discusses the four essential biomolecules for life: carbohydrates, lipids, proteins, and nucleic acids, highlighting their structures, functions, and significance in nutrition. It provides detailed descriptions of their building blocks, energy contributions, and examples of each type, along with specific roles they play in living organisms. Additionally, it includes information about the types of forces that affect these biomolecules and their organization in biological systems.