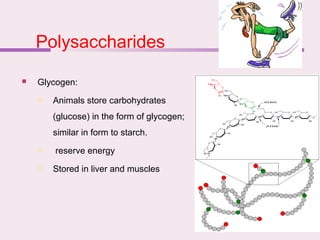
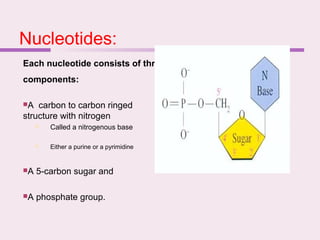
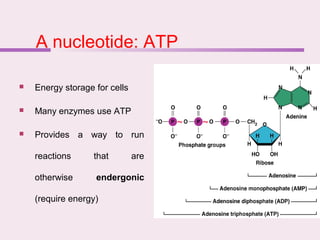
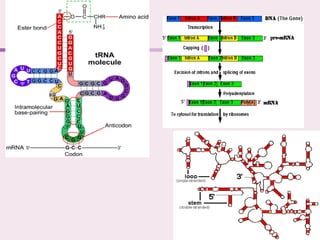
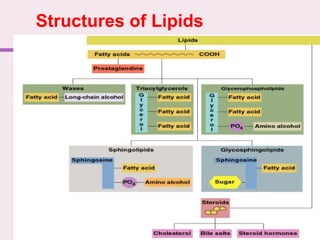
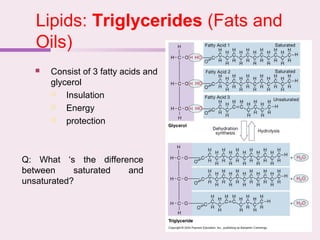
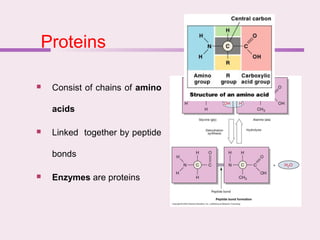
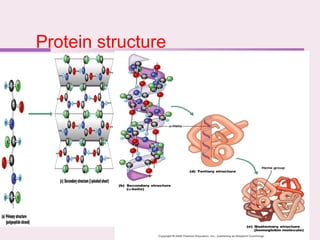
The document discusses the key biomolecules that make up living cells. It describes the four main types of biological macromolecules as carbohydrates, lipids, proteins, and nucleic acids. Carbohydrates include sugars and starches, and serve functions like energy storage. Lipids are insoluble in water and include fats, oils, and phospholipids. Proteins are made of amino acid chains and perform diverse roles such as structure, enzymes, transport. Nucleic acids DNA and RNA contain the genetic code and control protein synthesis. Each biomolecule has distinct but vital functions that allow cells and organisms to survive.