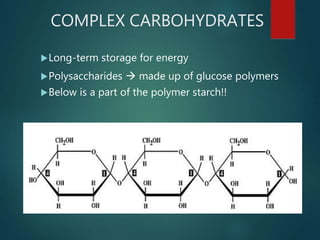
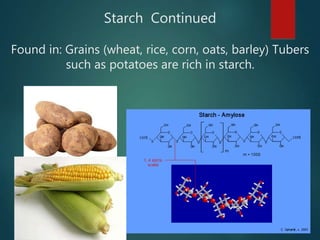
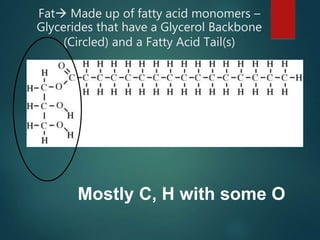
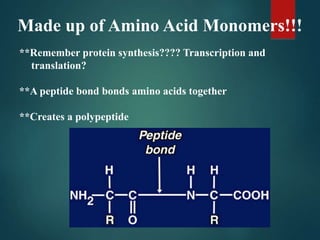
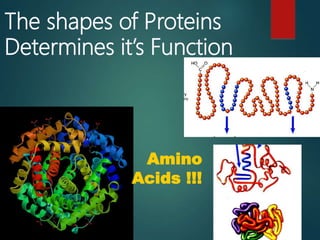
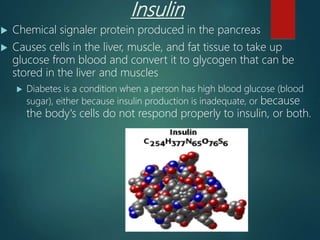
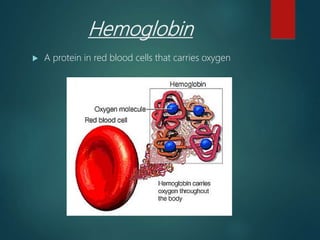
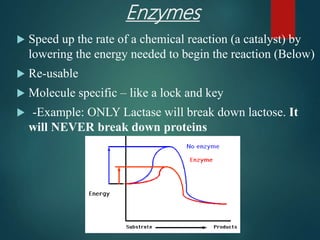
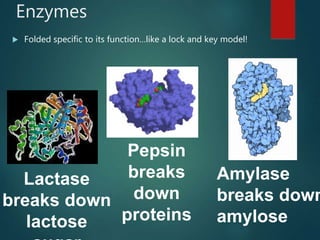
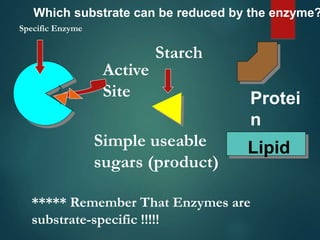
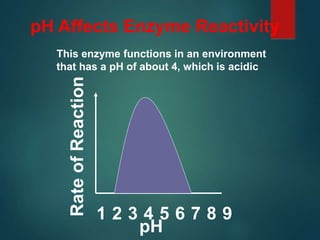
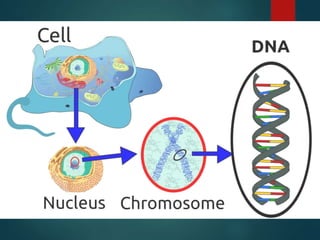
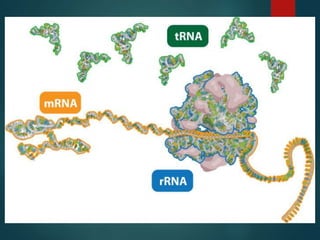
There are four classes of large biological molecules: proteins, lipids, carbohydrates, and nucleic acids. They are all organic and contain carbon, hydrogen, and oxygen. Carbohydrates include sugars and starches, lipids are used for energy storage and insulation, proteins perform structural and functional roles as enzymes and antibodies, and nucleic acids store and transmit genetic information as DNA and RNA.