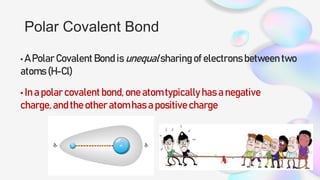
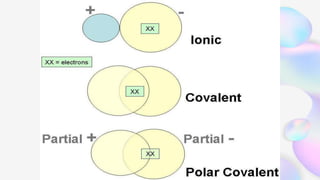
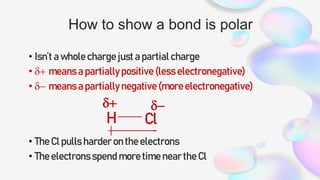
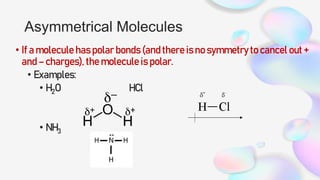
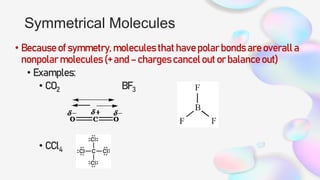
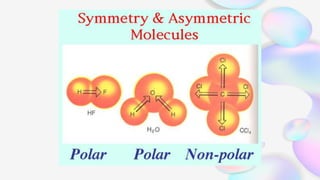
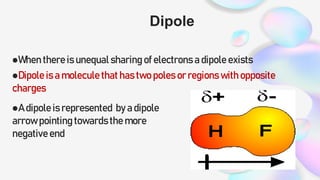
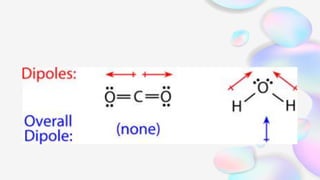
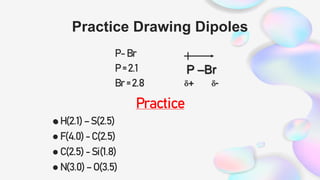
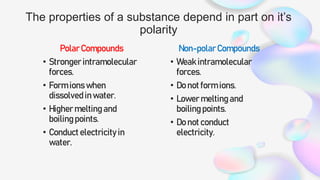
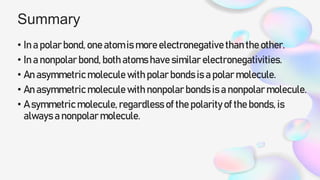
This document discusses polar and nonpolar molecules. It defines polarity as separation of electric charge leading to a molecule having a partial positive and negative end. Polar bonds form when electrons are shared unequally between atoms, while nonpolar bonds form when electrons are shared equally. Whether a molecule is polar or nonpolar depends on whether it has any polar bonds and if it has symmetrical charge distribution. Polar molecules are asymmetrical with polar bonds and have higher melting/boiling points, while nonpolar molecules have symmetrical or equal charge distribution. The document provides examples and exercises to classify different types of bonds and molecules.